Abstract
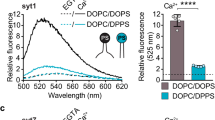
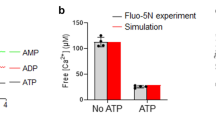
Synaptotagmin acts as a Ca2+ sensor in neurotransmitter release through its two C2 domains. Ca2+-dependent phospholipid binding is key for synaptotagmin function, but it is unclear how this activity cooperates with the SNARE complex involved in release or why Ca2+ binding to the C2B domain is more crucial for release than Ca2+ binding to the C2A domain. Here we show that Ca2+ induces high-affinity simultaneous binding of synaptotagmin to two membranes, bringing them into close proximity. The synaptotagmin C2B domain is sufficient for this ability, which arises from the abundance of basic residues around its surface. We propose a model wherein synaptotagmin cooperates with the SNAREs in bringing the synaptic vesicle and plasma membranes together and accelerates membrane fusion through the highly positive electrostatic potential of its C2B domain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sudhof, T.C. The synaptic vesicle cycle. Annu. Rev. Neurosci. 27, 509–547 (2004).
Chapman, E.R. Synaptotagmin: a Ca(2+) sensor that triggers exocytosis? Nat. Rev. Mol. Cell Biol. 3, 498–508 (2002).
Sutton, R.B., Davletov, B.A., Berghuis, A.M., Sudhof, T.C. & Sprang, S.R. Structure of the first C2 domain of synaptotagmin I: a novel Ca2+/phospholipid-binding fold. Cell 80, 929–938 (1995).
Shao, X., Fernandez, I., Sudhof, T.C. & Rizo, J. Solution structures of the Ca2+-free and Ca2+-bound C2A domain of synaptotagmin I: does Ca2+ induce a conformational change? Biochemistry 37, 16106–16115 (1998).
Fernandez, I. et al. Three-dimensional structure of the synaptotagmin 1 c(2)b-domain. Synaptotagmin 1 as a phospholipid binding machine. Neuron 32, 1057–1069 (2001).
Shao, X., Davletov, B.A., Sutton, R.B., Sudhof, T.C. & Rizo, J. Bipartite Ca2+-binding motif in C2 domains of synaptotagmin and protein kinase C. Science 273, 248–251 (1996).
Ubach, J., Zhang, X., Shao, X., Sudhof, T.C. & Rizo, J. Ca2+ binding to synaptotagmin: how many Ca2+ ions bind to the tip of a C2-domain? EMBO J. 17, 3921–3930 (1998).
Davletov, B.A. & Sudhof, T.C. A single C2 domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid binding. J. Biol. Chem. 268, 26386–26390 (1993).
Chapman, E.R. & Davis, A.F. Direct interaction of a Ca2+-binding loop of synaptotagmin with lipid bilayers. J. Biol. Chem. 273, 13995–14001 (1998).
Zhang, X., Rizo, J. & Sudhof, T.C. Mechanism of phospholipid binding by the C2A-domain of synaptotagmin I. Biochemistry 37, 12395–12403 (1998).
Fernandez-Chacon, R. et al. Synaptotagmin I functions as a calcium regulator of release probability. Nature 410, 41–49 (2001).
Rhee, J.S. et al. Augmenting neurotransmitter release by enhancing the apparent Ca2+-affinity of synaptotagmin 1. Proc. Natl. Acad. Sci. USA 102, 18664–18669 (2005).
Shin, O.H., Rizo, J. & Sudhof, T.C. Synaptotagmin function in dense core vesicle exocytosis studied in cracked PC12 cells. Nat. Neurosci. 5, 649–656 (2002).
Arac, D., Murphy, T. & Rizo, J. Facile detection of protein-protein interactions by one-dimensional NMR spectroscopy. Biochemistry 42, 2774–2780 (2003).
Shin, O.H. et al. Sr2+ binding to the Ca2+ binding site of the synaptotagmin 1 C2B domain triggers fast exocytosis without stimulating SNARE interactions. Neuron 37, 99–108 (2003).
Hanson, P.I., Roth, R., Morisaki, H., Jahn, R. & Heuser, J.E. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell 90, 523–535 (1997).
Lin, R.C. & Scheller, R.H. Structural organization of the synaptic exocytosis core complex. Neuron 19, 1087–1094 (1997).
Poirier, M.A. et al. The synaptic SNARE complex is a parallel four-stranded helical bundle. Nat. Struct. Biol. 5, 765–769 (1998).
Sutton, R.B., Fasshauer, D., Jahn, R. & Brunger, A.T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395, 347–353 (1998).
Weber, T. et al. SNAREpins: minimal machinery for membrane fusion. Cell 92, 759–772 (1998).
Hu, K. et al. Vesicular restriction of synaptobrevin suggests a role for calcium in membrane fusion. Nature 415, 646–650 (2002).
Kweon, D.H., Kim, C.S. & Shin, Y.K. Regulation of neuronal SNARE assembly by the membrane. Nat. Struct. Biol. 10, 440–447 (2003).
Sorensen, J.B. et al. The SNARE protein SNAP-25 is linked to fast calcium triggering of exocytosis. Proc. Natl. Acad. Sci. USA 99, 1627–1632 (2002).
Chen, X., Tang, J., Sudhof, T.C. & Rizo, J. Are neuronal SNARE proteins Ca2+ sensors? J. Mol. Biol. 347, 145–158 (2005).
Rickman, C. et al. Synaptotagmin interaction with the syntaxin/SNAP-25 dimer is mediated by an evolutionarily conserved motif and is sensitive to inositol hexakisphosphate. J. Biol. Chem. 279, 12574–12579 (2004).
Chapman, E.R., Desai, R.C., Davis, A.F. & Tornehl, C.K. Delineation of the oligomerization, AP-2 binding, and synprint binding region of the C2B domain of synaptotagmin. J. Biol. Chem. 273, 32966–32972 (1998).
Garcia, R.A., Forde, C.E. & Godwin, H.A. Calcium triggers an intramolecular association of the C2 domains in synaptotagmin. Proc. Natl. Acad. Sci. USA 97, 5883–5888 (2000).
Ubach, J. et al. The C2B domain of synaptotagmin I is a Ca2+-binding module. Biochemistry 40, 5854–5860 (2001).
Mackler, J.M., Drummond, J.A., Loewen, C.A., Robinson, I.M. & Reist, N.E. The C(2)B Ca(2+)-binding motif of synaptotagmin is required for synaptic transmission in vivo. Nature 418, 340–344 (2002).
Robinson, I.M., Ranjan, R. & Schwarz, T.L. Synaptotagmins I and IV promote transmitter release independently of Ca(2+) binding in the C(2)A domain. Nature 418, 336–340 (2002).
Fernandez-Chacon, R. et al. Structure/function analysis of Ca2+ binding to the C2A domain of synaptotagmin 1. J. Neurosci. 22, 8438–8446 (2002).
Nishiki, T. & Augustine, G.J. Dual roles of the C2B domain of synaptotagmin I in synchronizing Ca2+-dependent neurotransmitter release. J. Neurosci. 24, 8542–8550 (2004).
Wu, Y. et al. Visualization of synaptotagmin I oligomers assembled onto lipid monolayers. Proc. Natl. Acad. Sci. USA 100, 2082–2087 (2003).
Bai, J., Wang, P. & Chapman, E.R. C2A activates a cryptic Ca(2+)-triggered membrane penetration activity within the C2B domain of synaptotagmin I. Proc. Natl. Acad. Sci. USA 99, 1665–1670 (2002).
Gerber, S.H., Rizo, J. & Sudhof, T.C. Role of electrostatic and hydrophobic interactions in ca(2+)-dependent phospholipid binding by the c(2)a-domain from synaptotagmin I. Diabetes 51 Suppl 1, S12–S18 (2002).
Rufener, E., Frazier, A.A., Wieser, C.M., Hinderliter, A. & Cafiso, D.S. Membrane-bound orientation and position of the synaptotagmin C2B domain determined by site-directed spin labeling. Biochemistry 44, 18–28 (2005).
Frazier, A.A., Roller, C.R., Havelka, J.J., Hinderliter, A. & Cafiso, D.S. Membrane-bound orientation and position of the synaptotagmin I C2A domain by site-directed spin labeling. Biochemistry 42, 96–105 (2003).
Crowley, K.S., Reinhart, G.D. & Johnson, A.E. The signal sequence moves through a ribosomal tunnel into a noncytoplasmic aqueous environment at the ER membrane early in translocation. Cell 73, 1101–1115 (1993).
Wu, P. & Brand, L. Resonance energy transfer: methods and applications. Anal. Biochem. 218, 1–13 (1994).
Bai, J., Tucker, W.C. & Chapman, E.R. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat. Struct. Mol. Biol. 11, 36–44 (2004).
Schiavo, G., Matteoli, M. & Montecucco, C. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 80, 717–766 (2000).
Tucker, W.C., Weber, T. & Chapman, E.R. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science 304, 435–438 (2004).
Chen, X. et al. SNARE mediated lipid mixing depends on the physical state of the vesicles Biophys. J published online 16 December 2005 (10.1529/biophysj.105.071415).
Chernomordik, L.V., Melikyan, G.B. & Chizmadzhev, Y.A. Biomembrane fusion: a new concept derived from model studies using two interacting planar lipid bilayers. Biochim. Biophys. Acta 906, 309–352 (1987).
Hartmann, W. & Galla, H.J. Binding of polylysine to charged bilayer membranes: molecular organization of a lipid.peptide complex. Biochim. Biophys. Acta 509, 474–490 (1978).
Borden, C.R., Stevens, C.F., Sullivan, J.M. & Zhu, Y. Synaptotagmin mutants Y311N and K326/327A alter the calcium dependence of neurotransmission. Mol. Cell. Neurosci. 29, 462–470 (2005).
Ludtke, S.J., Jakana, J., Song, J.L., Chuang, D.T. & Chiu, W. A 11.5 A single particle reconstruction of GroEL using EMAN. J. Mol. Biol. 314, 253–262 (2001).
Kremer, J.R., Mastronarde, D.N. & McIntosh, J.R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116, 71–76 (1996).
Nicholls, A., Sharp, K.A. & Honig, B. Protein folding and association—insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins Struct. Funct. Genet. 11, 281–296 (1991).
Acknowledgements
We thank T. Kodadek and L. Burdine for assistance with the cross-linking experiments, M. Schmid for assistance on the three-dimensional tomographic reconstruction and N. Mizuno and Z. Metlagel for help with the negative-staining experiments. This work was supported by grants from the Welch Foundation (to A.E.J. and J.R.) and by US National Institutes of Health grants P41RR02250 (to W.C.), GM26494 (to A.E.J.) and NS40944 (to J.R.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Problems with labeling C277 of synaptotagmin 1 (PDF 690 kb)
Supplementary Fig. 2
Reconstruction of the oligomeric model of synaptotagmin 1 (PDF 618 kb)
Supplementary Fig. 3
Negative stain em analysis of synpatotagmin/calcium/lipid samples (PDF 1135 kb)
Supplementary Fig. 4
Synaptotagmin does not oligomerize (PDF 226 kb)
Supplementary Fig. 5
Calcium dependence of synaptotagmin induced vesicle clustering (PDF 77 kb)
Supplementary Fig. 6
Diverse considerations supporting the model of Figure 6 (PDF 256 kb)
Supplementary Video 1
Tomographic 3D reconstruction of a vesicle cluster (MPG 2310 kb)
Rights and permissions
About this article
Cite this article
Araç, D., Chen, X., Khant, H. et al. Close membrane-membrane proximity induced by Ca2+-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat Struct Mol Biol 13, 209–217 (2006). https://doi.org/10.1038/nsmb1056
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb1056
This article is cited by
-
A first-passage approach to diffusion-influenced reversible binding and its insights into nanoscale signaling at the presynapse
Scientific Reports (2021)
-
Lysine acetylation regulates the interaction between proteins and membranes
Nature Communications (2021)
-
Function of Drosophila Synaptotagmins in membrane trafficking at synapses
Cellular and Molecular Life Sciences (2021)
-
Evaluation of the tert-butyl group as a probe for NMR studies of macromolecular complexes
Journal of Biomolecular NMR (2021)
-
Analysis of asymmetry in lipid and content mixing assays with reconstituted proteoliposomes containing the neuronal SNAREs
Scientific Reports (2020)