Key Points
-
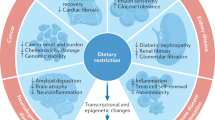
Dietary restriction — the limitation of available nutrients without malnutrition — can extend the lifespan of every species that has been tested, including mammals. Dietary restriction also reduces the incidence of and/or slows the progression of many age-related pathologies.
-
Dietary restriction has been modelled in yeast by reducing glucose concentration in the medium from 2% to 0.5% (moderate dietary restriction). Studies using this system have revealed a genetic pathway that controls dietary-restriction-induced longevity, which is dependent on increased mitochondrial respiration and sirtuin 2 (SIR2) genes.
-
A more severe form of dietary restriction in yeast, 0.05% glucose (severe dietary restriction), causes lifespan extension that is not dependent on SIR2 genes or on increased mitochondrial respiration. Instead, this form of dietary restriction may function through reduced target of rapamycin (TOR) and/or AKT signalling.
-
Few genes that mediate metazoan dietary restriction have been identified, but candidate genes that are known to be involved in both lifespan control and nutrient sensing include those encoding TOR, AMP-dependent protein kinase (AMPK), insulin signalling proteins and sirtuins.
-
Genes that have been clearly identified as required for metazoan dietary-restriction-induced longevity include skn-1 and pha-4 (in worms), Sir2 (in flies) and the growth hormone receptor gene (in mice).
-
Invertebrate studies have shown that neurons are crucial mediators of the dietary-restriction response in worms and flies.
-
A possible role of the mammalian hypothalamus in the response to dietary restriction is proposed in this Review. This is suggested by the clear demonstration of the importance of central neuronal signalling for dietary restriction in lower organisms and the many parallels between the genetics of invertebrate lifespan control and the genetics of hypothalamic energy sensing, as well as data suggesting that central hormonal axes under hypothalamic control have a role in mammalian dietary restriction.
Abstract
Caloric restriction is the only known non-genetic intervention that robustly extends lifespan in mammals. This regimen also attenuates the incidence and progression of many age-dependent pathologies. Understanding the genetic mechanisms that underlie dietary-restriction-induced longevity would therefore have profound implications for future medical treatments aimed at tackling conditions that are associated with the ageing process. Until recently, however, almost nothing was known about these mechanisms in metazoans. Recent advances in our understanding of the genetic bases of energy sensing and lifespan control in yeast, invertebrates and mammals have begun to solve this puzzle. Evidence is mounting that the brain has a crucial role in sensing dietary restriction and promoting longevity in metazoans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lin, S. J. et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature 418, 344–348 (2002). The first demonstration that DR increases respiration as an essential step in longevity.
Klass, M. R. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech. Ageing Dev. 6, 413–429 (1977).
Loeb, J. & Northrop, J. H. On the influence of food and temperature upon the duration of life. J. Biol. Chem. 32, 103–121 (1917).
McCay, C. M., Crowell, M. F. & Maynard, L. A. The effect of retarded growth upon the length of life span and upon the ultimate body size. J. Nutr. 10, 63–79 (1935).
Mattison, J. A., Roth, G. S., Lane, M. A. & Ingram, D. K. Dietary restriction in aging nonhuman primates. Interdiscip. Top. Gerontol. 35, 137–158 (2007).
Mattson, M. P. & Wan, R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J. Nutr. Biochem. 16, 129–137 (2005).
Klebanov, S. Can short-term dietary restriction and fasting have a long-term anticarcinogenic effect? Interdiscip. Top. Gerontol. 35, 176–192 (2007).
Wang, J. et al. Caloric restriction attenuates β-amyloid neuropathology in a mouse model of Alzheimer's disease. FASEB J. 19, 659–661 (2005).
Maswood, N. et al. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson's disease. Proc. Natl Acad. Sci. USA 101, 18171–18176 (2004).
Anson, R. M. et al. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc. Natl Acad. Sci. USA 100, 6216–6220 (2003).
Koubova, J. & Guarente, L. How does calorie restriction work? Genes Dev. 17, 313–321 (2003).
Gems, D., Pletcher, S. & Partridge, L. Interpreting interactions between treatments that slow aging. Aging Cell 1, 1–9 (2002).
Lin, S. J., Defossez, P. A. & Guarente, L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 289, 2126–2128 (2000). The first identification of genes that are required for DR longevity in yeast.
Smith, E. D., Kennedy, B. K. & Kaeberlein, M. Genome-wide identification of conserved longevity genes in yeast and worms. Mech. Ageing Dev. 128, 106–111 (2007).
Guarente, L. & Kenyon, C. Genetic pathways that regulate ageing in model organisms. Nature 408, 255–262 (2000).
Kaeberlein, M., Kirkland, K. T., Fields, S. & Kennedy, B. K. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2, e296 (2004). The first report that yeast DR proceeds independently of SIR2 under some circumstances.
Kaeberlein, M. et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 310, 1193–1196 (2005).
Easlon, E. et al. The dihydrolipoamide acetyltransferase is a novel metabolic longevity factor and is required for calorie restriction-mediated life span extension. J. Biol. Chem. 282, 6161–6171 (2007).
Lin, S. J., Ford, E., Haigis, M., Liszt, G. & Guarente, L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes. Dev. 18, 12–16 (2004).
Anderson, R. M., Bitterman, K. J., Wood, J. G., Medvedik, O. & Sinclair, D. A. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature 423, 181–185 (2003).
Lamming, D. W. et al. HST2 mediates SIR2-independent life-span extension by calorie restriction. Science 309, 1861–1864 (2005).
Kaeberlein, M. et al. Comment on “HST2 mediates SIR2-independent life-span extension by calorie restriction”. Science 312, 1312 (2006).
Tsuchiya, M. et al. Sirtuin-independent effects of nicotinamide on lifespan extension from calorie restriction in yeast. Aging Cell 5, 505–514 (2006).
Longo, V. D. & Kennedy, B. K. Sirtuins in aging and age-related disease. Cell 126, 257–268 (2006).
Sinclair, D. A. & Guarente, L. Extrachromosomal rDNA circles — a cause of aging in yeast. Cell 91, 1033–1042 (1997).
Johnson, F. B., Sinclair, D. A. & Guarente, L. Molecular biology of aging. Cell 96, 291–302 (1999).
Guarente, L. SIR2 and aging — the exception that proves the rule. Trends Genet. 17, 391–392 (2001).
Kaeberlein, M. et al. Increased life span due to calorie restriction in respiratory-deficient yeast. PLoS Genet. 1, e69 (2005).
Lin, S. J. & Guarente, L. Increased life span due to calorie restriction in respiratory-deficient yeast. PLoS Genet. 2, e33 (2006).
Powers, R. W. 3rd, Kaeberlein, M., Caldwell, S. D., Kennedy, B. K. & Fields, S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 20, 174–184 (2006).
Fabrizio, P. et al. Sir2 blocks extreme life-span extension. Cell 123, 655–667 (2005).
Bishop, N. A. & Guarente, L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature 447, 545–549 (2007). The first demonstration of a central role of neuronal signalling in DR, resulting in increased respiration and longevity.
Nisoli, E. et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310, 314–317 (2005). This work demonstrated that DR in mice causes increased mitochondrial function and involves nitric oxide and Sirt1.
Houthoofd, K. et al. Axenic growth up-regulates mass-specific metabolic rate, stress resistance, and extends life span in Caenorhabditis elegans. Exp. Gerontol. 37, 1371–1378 (2002).
Houthoofd, K. et al. No reduction of metabolic rate in food restricted Caenorhabditis elegans. Exp. Gerontol. 37, 1359–1369 (2002).
Rogina, B. & Helfand, S. L. SIR2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl Acad. Sci. USA 101, 15998–16003 (2004). First report of a gene required for DR longevity in the fly.
Chen, D., Steele, A. D., Lindquist, S. & Guarente, L. Increase in activity during calorie restriction requires Sirt1. Science 310, 1641 (2005).
Viswanathan, M., Kim, S. K., Berdichevsky, A. & Guarente, L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev. Cell 9, 605–615 (2005).
Tissenbaum, H. A. & Guarente, L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410, 227–230 (2001).
Hansen, M. et al. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell 6, 95–110 (2007).
Lee, G. D. et al. Dietary deprivation extends lifespan in Caenorhabditis elegans. Aging Cell 5, 515–524 (2006).
Kaeberlein, T. L. et al. Lifespan extension in Caenorhabditis elegans by complete removal of food. Aging Cell 5, 487–494 (2006).
Wang, Y. & Tissenbaum, H. A. Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO. Mech. Ageing Dev. 127, 48–56 (2006).
Vellai, T. et al. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature 426, 620 (2003).
Kapahi, P. et al. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol. 14, 885–890 (2004).
Kenyon, C. The plasticity of aging: insights from long-lived mutants. Cell 120, 449–460 (2005).
Houthoofd, K., Braeckman, B. P., Johnson, T. E. & Vanfleteren, J. R. Life extension via dietary restriction is independent of the Ins/IGF-1 signalling pathway in Caenorhabditis elegans. Exp. Gerontol. 38, 947–954 (2003).
Lakowski, B. & Hekimi, S. The genetics of caloric restriction in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 95, 13091–13096 (1998). The first report of a gene required for the DR response in worms.
Apfeld, J., O'Connor, G., McDonagh, T., DiStefano, P. S. & Curtis, R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 18, 3004–3009 (2004).
Curtis, R., O'Connor, G. & DiStefano, P. S. Aging networks in Caenorhabditis elegans: AMP-activated protein kinase (aak-2) links multiple aging and metabolism pathways. Aging Cell 5, 119–126 (2006).
Ashrafi, K., Lin, S. S., Manchester, J. K. & Gordon, J. I. Sip2p and its partner snf1p kinase affect aging in S. cerevisiae. Genes Dev. 14, 1872–1885 (2000).
Kenyon, C., Chang, J., Gensch, E., Rudner, A. & Tabtiang, R. A C. elegans mutant that lives twice as long as wild type. Nature 366, 461–464 (1993).
Libina, N., Berman, J. R. & Kenyon, C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell 115, 489–502 (2003).
Wolkow, C. A., Kimura, K. D., Lee, M. S. & Ruvkun, G. Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science 290, 147–150 (2000).
Panowski, S. H., Wolff, S., Aguilaniu, H., Durieux, J. & Dillin, A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature 447, 550–555 (2007).
Clancy, D. J., Gems, D., Hafen, E., Leevers, S. J. & Partridge, L. Dietary restriction in long-lived dwarf flies. Science 296, 319 (2002). An instructive report that introduces the importance of testing a range of food levels in DR interaction studies.
Tatar, M. Diet restriction in Drosophila melanogaster. Design and analysis. Interdiscip. Top. Gerontol. 35, 115–136 (2007).
Clancy, D. J. et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 292, 104–106 (2001).
Partridge, L., Piper, M. D. & Mair, W. Dietary restriction in Drosophila. Mech. Ageing Dev. 126, 938–950 (2005).
Flurkey, K., Papaconstantinou, J., Miller, R. A. & Harrison, D. E. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc. Natl. Acad. Sci. USA 98, 6736–6741 (2001).
Brown-Borg, H. M., Borg, K. E., Meliska, C. J. & Bartke, A. Dwarf mice and the ageing process. Nature 384, 33 (1996).
Bartke, A. et al. Extending the lifespan of long-lived mice. Nature 414, 412 (2001).
Coschigano, K. T., Clemmons, D., Bellush, L. L. & Kopchick, J. J. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology 141, 2608–2613 (2000).
Bonkowski, M. S., Rocha, J. S., Masternak, M. M., Al Regaiey, K. A. & Bartke, A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc. Natl Acad. Sci. USA 103, 7901–7905 (2006). The first report of a mammalian mutant that is unresponsive to DR.
Apfeld, J. & Kenyon, C. Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature 402, 804–809 (1999).
Alcedo, J. & Kenyon, C. Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron 41, 45–55 (2004).
Broughton, S. J. et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc. Natl Acad. Sci. USA 102, 3105–3110 (2005).
Libert, S. et al. Regulation of Drosophila life span by olfaction and food-derived odors. Science 315, 1133–1137 (2007). The first indication that sensory perception is involved in DR longevity in metazoans.
Fridell, Y. W., Sanchez-Blanco, A., Silvia, B. A. & Helfand, S. L. Targeted expression of the human uncoupling protein 2 (hUCP2) to adult neurons extends life span in the fly. Cell Metab. 1, 145–152 (2005).
Sanchez-Blanco, A., Fridell, Y. W. & Helfand, S. L. Involvement of Drosophila uncoupling protein 5 in metabolism and aging. Genetics 172, 1699–1710 (2006).
Bargmann, C. I. & Horvitz, H. R. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 7, 729–742 (1991).
Bargmann, C. I. & Horvitz, H. R. Control of larval development by chemosensory neurons in Caenorhabditis elegans. Science 251, 1243–1246 (1991).
Riddle, D. L. & Albert, P. S. in C. elegans II (eds Riddle, D. L., Blumenthal, T., Meyer, B. J. & Preiss, J. R.) 739–768 (Cold Spring Harbor Laboratory Press, Plainview, 1997).
Flier, J. S. Obesity wars: molecular progress confronts an expanding epidemic. Cell 116, 337–350 (2004).
Lam, T. K., Schwartz, G. J. & Rossetti, L. Hypothalamic sensing of fatty acids. Nature Neurosci. 8, 579–584 (2005).
Levin, B. E., Routh, V. H., Kang, L., Sanders, N. M. & Dunn-Meynell, A. A. Neuronal glucosensing: what do we know after 50 years? Diabetes 53, 2521–2528 (2004).
Hara, J. et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 30, 345–354 (2001).
Shimada, M., Tritos, N. A., Lowell, B. B., Flier, J. S. & Maratos-Flier, E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature 396, 670–674 (1998).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994).
Ahima, R. S. et al. Role of leptin in the neuroendocrine response to fasting. Nature 382, 250–252 (1996).
Bruning, J. C. et al. Role of brain insulin receptor in control of body weight and reproduction. Science 289, 2122–2125 (2000).
Schwartz, M. W., Woods, S. C., Porte, D. Jr., Seeley, R. J. & Baskin, D. G. Central nervous system control of food intake. Nature 404, 661–671 (2000).
Kitamura, T. et al. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nature Med. 12, 534–540 (2006).
Niswender, K. D. et al. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes 52, 227–231 (2003).
Niswender, K. D. et al. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature 413, 794–795 (2001).
Plum, L., Belgardt, B. F. & Bruning, J. C. Central insulin action in energy and glucose homeostasis. J. Clin. Invest. 116, 1761–1766 (2006).
Minokoshi, Y. et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428, 569–574 (2004).
Cota, D. et al. Hypothalamic mTOR signaling regulates food intake. Science 312, 927–930 (2006).
Ibrahim, N. et al. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology 144, 1331–1340 (2003).
Muroya, S., Yada, T., Shioda, S. & Takigawa, M. Glucose-sensitive neurons in the rat arcuate nucleus contain neuropeptide Y. Neurosci. Lett. 264, 113–116 (1999).
Eto, K. et al. Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science 283, 981–985 (1999).
Mobbs, C. V. et al. Secrets of the lac operon. Glucose hysteresis as a mechanism in dietary restriction, aging and disease. Interdiscip. Top. Gerontol. 35, 39–68 (2007).
Yang, X. J., Kow, L. M., Pfaff, D. W. & Mobbs, C. V. Metabolic pathways that mediate inhibition of hypothalamic neurons by glucose. Diabetes 53, 67–73 (2004).
Coppola, A. et al. A central thermogenic-like mechanism in feeding regulation: an interplay between arcuate nucleus T3 and UCP2. Cell Metab. 5, 21–33 (2007).
Conti, B. et al. Transgenic mice with a reduced core body temperature have an increased life span. Science 314, 825–828 (2006). This paper shows that expression of a transgene in a single hypothalamic neuron type is sufficient to extend murine lifespan, and suggests that the mechanism of lifespan extension overlaps with that of DR.
Harrison, D. E., Archer, J. R. & Astle, C. M. Effects of food restriction on aging: separation of food intake and adiposity. Proc. Natl Acad. Sci. USA 81, 1835–1838 (1984).
Weindruch, R. & Walford, R. L. The Retardation of Aging and Disease by Dietary Restriction (Charles C. Thomas Pub, Springfield, 1988).
Martin, B. et al. Sex-dependent metabolic, neuroendocrine and cognitive responses to dietary energy restriction and excess. Endocrinology 148, 4318–4333 (2007).
Mobbs, C. V. et al. Neuroendocrine and pharmacological manipulations to assess how caloric restriction increases life span. J. Gerontol. A Biol. Sci. Med. Sci. 56, S34–S44 (2001).
Sohal, R. S. & Weindruch, R. Oxidative stress, caloric restriction, and aging. Science 273, 59–63 (1996).
Acknowledgements
We thank members of the scientific community working on ageing and acknowledge support from the US National Institutes of Health and the Glenn Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Leonard Guarente is a founder of Elixir Pharmaceuticals.
Glossary
- Feeding ad libidum
-
A feeding protocol in which an organism is allowed to eat as much as desired, given an unlimited food supply.
- Hypothalamus
-
A region of the brain that regulates organismal homeostasis, in particular energy balance, by regulating behaviour and metabolism by endocrine and autonomic signalling.
- Uncoupling proteins
-
A family of mammalian proteins that dissipate the proton gradient across the mitochondrial membrane, necessitating more rapid respiration to maintain the rate of ATP production.
- Dauer
-
An alternative larval stage of Caenorhabditis elegans that is adapted to survive adverse conditions.
Rights and permissions
About this article
Cite this article
Bishop, N., Guarente, L. Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nat Rev Genet 8, 835–844 (2007). https://doi.org/10.1038/nrg2188
Issue Date:
DOI: https://doi.org/10.1038/nrg2188
This article is cited by
-
Adaptive responses of yeast strains tolerant to acidic pH, acetate, and supraoptimal temperature
Applied Microbiology and Biotechnology (2023)
-
Several areas of overlap between obesity and aging indicate obesity as a biomarker of accelerated aging of human B cell function and antibody responses
Immunity & Ageing (2022)
-
Molecular mechanisms of exceptional lifespan increase of Drosophila melanogaster with different genotypes after combinations of pro-longevity interventions
Communications Biology (2022)
-
SIRT7 antagonizes human stem cell aging as a heterochromatin stabilizer
Protein & Cell (2020)
-
Effects of short-term fasting on cancer treatment
Journal of Experimental & Clinical Cancer Research (2019)