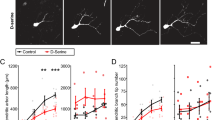
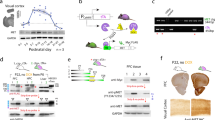
Abstract
Functional maturation of GABAergic innervation in the developing visual cortex is regulated by neural activity and sensory inputs and in turn influences the critical period of ocular dominance plasticity. Here we show that polysialic acid (PSA), presented by the neural cell adhesion molecule, has a role in the maturation of GABAergic innervation and ocular dominance plasticity. Concentrations of PSA significantly decline shortly after eye opening in the adolescent mouse visual cortex; this decline is hindered by visual deprivation. The developmental and activity-dependent regulation of PSA expression is inversely correlated with the maturation of GABAergic innervation. Premature removal of PSA in visual cortex results in precocious maturation of perisomatic innervation by basket interneurons, enhanced inhibitory synaptic transmission, and earlier onset of ocular dominance plasticity. The developmental and activity-dependent decline of PSA expression therefore regulates the timing of the maturation of GABAergic inhibition and the onset of ocular dominance plasticity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Rothbard, J.B., Brackenbury, R., Cunningham, B.A. & Edelman, G.M. Differences in the carbohydrate structures of neural cell-adhesion molecules from adult and embryonic chicken brains. J. Biol. Chem. 257, 11064–11069 (1982).
Johnson, C.P., Fujimoto, I., Rutishauser, U. & Leckband, D.E. Direct evidence that neural cell adhesion molecule (NCAM) polysialylation increases intermembrane repulsion and abrogates adhesion. J. Biol. Chem. 280, 137–145 (2005).
Acheson, A., Sunshine, J.L. & Rutishauser, U. NCAM polysialic acid can regulate both cell-cell and cell-substrate interactions. J. Cell Biol. 114, 143–153 (1991).
Fujimoto, I., Bruses, J.L. & Rutishauser, U. Regulation of cell adhesion by polysialic acid. Effects on cadherin, immunoglobulin cell adhesion molecule and integrin function and independence from neural cell adhesion molecule binding or signaling activity. J. Biol. Chem. 276, 31745–31751 (2001).
Ono, K., Tomasiewicz, H., Magnuson, T. & Rutishauser, U. N-CAM mutation inhibits tangential neuronal migration and is phenocopied by enzymatic removal of polysialic acid. Neuron 13, 595–609 (1994).
Nait-Oumesmar, B. et al. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur. J. Neurosci. 11, 4357–4366 (1999).
O'Leary, D.D. & Terashima, T. Cortical axons branch to multiple subcortical targets by interstitial axon budding: implications for target recognition and 'waiting periods'. Neuron 1, 901–910 (1988).
Seki, T. & Rutishauser, U. Removal of polysialic acid-neural cell adhesion molecule induces aberrant mossy fiber innervation and ectopic synaptogenesis in the hippocampus. J. Neurosci. 18, 3757–3766 (1998).
Tang, J., Landmesser, L. & Rutishauser, U. Polysialic acid influences specific pathfinding by avian motoneurons. Neuron 8, 1031–1044 (1992).
Tang, J., Rutishauser, U. & Landmesser, L. Polysialic acid regulates growth cone behavior during sorting of motor axons in the plexus region. Neuron 13, 405–414 (1994).
Yamamoto, N. et al. Inhibitory mechanism by polysialic acid for lamina-specific branch formation of thalamocortical axons. J. Neurosci. 20, 9145–9151 (2000).
Yin, X., Watanabe, M. & Rutishauser, U. Effect of polysialic acid on the behavior of retinal ganglion cell axons during growth into the optic tract and tectum. Development 121, 3439–3446 (1995).
El Maarouf, A. & Rutishauser, U. Removal of polysialic acid induces aberrant pathways, synaptic vesicle distribution and terminal arborization of retinotectal axons. J. Comp. Neurol. 460, 203–211 (2003).
Dityatev, A. et al. Polysialylated neural cell adhesion molecule promotes remodeling and formation of hippocampal synapses. J. Neurosci. 24, 9372–9382 (2004).
Eckhardt, M. et al. Mice deficient in the polysialyltransferase ST8SiaIV/PST-1 allow discrimination of the roles of neural cell adhesion molecule protein and polysialic acid in neural development and synaptic plasticity. J. Neurosci. 20, 5234–5244 (2000).
Muller, D. et al. PSA-NCAM is required for activity-induced synaptic plasticity. Neuron 17, 413–422 (1996).
Theodosis, D.T., Bonhomme, R., Vitiello, S., Rougon, G. & Poulain, D.A. Cell surface expression of polysialic acid on NCAM is a prerequisite for activity-dependent morphological neuronal and glial plasticity. J. Neurosci. 19, 10228–10236 (1999).
Bruses, J.L. & Rutishauser, U. Roles, regulation and mechanism of polysialic acid function during neural development. Biochimie 83, 635–643 (2001).
Swadlow, H.A. Fast-spike interneurons and feedforward inhibition in awake sensory neocortex. Cereb. Cortex 13, 25–32 (2003).
Pouille, F. & Scanziani, M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science 293, 1159–1163 (2001).
Hasenstaub, A. et al. Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron 47, 423–435 (2005).
Somogyi, P. & Klausberger, T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J. Physiol. (Lond.) 562, 9–26 (2005).
Hensch, T.K. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 6, 877–888 (2005).
Hanover, J.L., Huang, Z.J., Tonegawa, S. & Stryker, M.P. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J. Neurosci. 19, RC40 (1999).
Fagiolini, M. & Hensch, T.K. Inhibitory threshold for critical-period activation in primary visual cortex. Nature 404, 183–186 (2000).
Markram, H. et al. Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 5, 793–807 (2004).
Chattopadhyaya, B. et al. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J. Neurosci. 24, 9598–9611 (2004).
Morales, B., Choi, S.Y. & Kirkwood, A. Dark rearing alters the development of GABAergic transmission in visual cortex. J. Neurosci. 22, 8084–8090 (2002).
Huang, Z.J. et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98, 739–755 (1999).
Chattopadhyaya, B. et al. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron 54, 889–903 (2007).
Toki, S. et al. Importance of early lighting conditions in maternal care by dam as well as anxiety and memory later in life of offspring. Eur. J. Neurosci. 25, 815–829 (2007).
Rutishauser, U., Watanabe, M., Silver, J., Troy, F.A. & Vimr, E.R. Specific alteration of NCAM-mediated cell adhesion by an endoneuraminidase. J. Cell Biol. 101, 1842–1849 (1985).
Klostermann, O. & Wahle, P. Patterns of spontaneous activity and morphology of interneuron types in organotypic cortex and thalamus-cortex cultures. Neuroscience 92, 1243–1259 (1999).
Di Cristo, G. et al. Subcellular domain–restricted GABAergic innervation in primary visual cortex in the absence of sensory and thalamic inputs. Nat. Neurosci. 7, 1184–1186 (2004).
Echevarria, D. & Albus, K. Activity-dependent development of spontaneous bioelectric activity in organotypic cultures of rat occipital cortex. Brain Res. Dev. Brain Res. 123, 151–164 (2000).
Dupuy, S.T. & Houser, C.R. Prominent expression of two forms of glutamate decarboxylase in the embryonic and early postnatal rat hippocampal formation. J. Neurosci. 16, 6919–6932 (1996).
Tian, N. et al. The role of the synthetic enzyme GAD65 in the control of neuronal γ-aminobutyric acid release. Proc. Natl. Acad. Sci. USA 96, 12911–12916 (1999).
Asada, H. et al. Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc. Natl. Acad. Sci. USA 94, 6496–6499 (1997).
Minelli, A., Alonso-Nanclares, L., Edwards, R.H., DeFelipe, J. & Conti, F. Postnatal development of the vesicular GABA transporter in rat cerebral cortex. Neuroscience 117, 337–346 (2003).
Frenkel, M.Y. & Bear, M.F. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron 44, 917–923 (2004).
Somogyi, P., Tamas, G., Lujan, R. & Buhl, E.H. Salient features of synaptic organization in the cerebral cortex. Brain Res. Brain Res. Rev. 26, 113–135 (1998).
Knott, G.W., Quairiaux, C., Genoud, C. & Welker, E. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron 34, 265–273 (2002).
Kurosawa, N., Yoshida, Y., Kojima, N. & Tsuji, S. Polysialic acid synthase (ST8Sia II/STX) mRNA expression in the developing mouse central nervous system. J. Neurochem. 69, 494–503 (1997).
Soares, S., von Boxberg, Y., Ravaille-Veron, M., Vincent, J.D. & Nothias, F. Morphofunctional plasticity in the adult hypothalamus induces regulation of polysialic acid–neural cell adhesion molecule through changing activity and expression levels of polysialyltransferases. J. Neurosci. 20, 2551–2557 (2000).
Angata, K. & Fukuda, M. Polysialyltransferases: major players in polysialic acid synthesis on the neural cell adhesion molecule. Biochimie 85, 195–206 (2003).
Bouzioukh, F., Tell, F., Jean, A. & Rougon, G. NMDA receptor and nitric oxide synthase activation regulate polysialylated neural cell adhesion molecule expression in adult brainstem synapses. J. Neurosci. 21, 4721–4730 (2001).
Bruses, J.L. & Rutishauser, U. Regulation of neural cell adhesion molecule polysialylation: evidence for nontranscriptional control and sensitivity to an intracellular pool of calcium. J. Cell Biol. 140, 1177–1186 (1998).
Muller, D. et al. Brain-derived neurotrophic factor restores long-term potentiation in polysialic acid–neural cell adhesion molecule-deficient hippocampus. Proc. Natl. Acad. Sci. USA 97, 4315–4320 (2000).
Angata, K. et al. Sialyltransferase ST8Sia-II assembles a subset of polysialic acid that directs hippocampal axonal targeting and promotes fear behavior. J. Biol. Chem. 279, 32603–32613 (2004).
Weinhold, B. et al. Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by deletion of the neural cell adhesion molecule. J. Biol. Chem. 280, 42971–42977 (2005).
Acknowledgements
We thank P. Wu, E. Putignano, N. Berardi and J. Boehm for technical assistance and discussion; and E. Ruthazer for critically reading the manuscript. This work was supported by the National Institutes of Health (RO1 EY 13564-01). G.D.C. has a National Association for Research in Schizophrenia and Depression (NARSAD) Young Investigator Award founded by the Forrest C. Lattner Foundation. Z.J.H. is a Pew and McKnight Scholar.
Author information
Authors and Affiliations
Contributions
G.D.C. and Z.J.H. conceived and organized the project and wrote the manuscript. G.D.C. conducted most of the experiments. B.C. contributed to the in vitro and in vivo morphological studies. S.J.K. and Y.F. carried out the mPSC recordings. M.-C.B. performed the western blotting. C.Z.W. contributed to the PSA expression analysis in vivo. U.R. provided endoN and advice. L.M. provided the facility for dark rearing and VEP recording.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplementary Methods (PDF 1496 kb)
Rights and permissions
About this article
Cite this article
Di Cristo, G., Chattopadhyaya, B., Kuhlman, S. et al. Activity-dependent PSA expression regulates inhibitory maturation and onset of critical period plasticity. Nat Neurosci 10, 1569–1577 (2007). https://doi.org/10.1038/nn2008
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn2008