Abstract
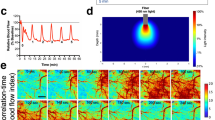
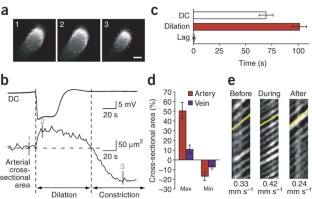
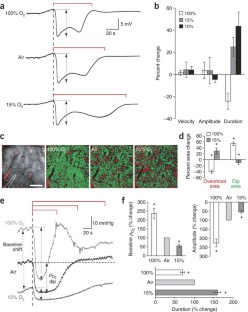
Cortical spreading depression (CSD) is a self-propagating wave of cellular depolarization that has been implicated in migraine and in progressive neuronal injury after stroke and head trauma. Using two-photon microscopic NADH imaging and oxygen sensor microelectrodes in live mouse cortex, we find that CSD is linked to severe hypoxia and marked neuronal swelling that can last up to several minutes. Changes in dendritic structures and loss of spines during CSD are comparable to those during anoxic depolarization. Increasing O2 availability shortens the duration of CSD and improves local redox state. Our results indicate that tissue hypoxia associated with CSD is caused by a transient increase in O2 demand exceeding vascular O2 supply.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Leao, A. Spreading depression of activity in cerebral cortex. J. Neurophysiol. 7, 359–390 (1944).
Martins-Ferreira, H., Nedergaard, M. & Nicholson, C. Perspectives on spreading depression. Brain Res. Brain Res. Rev. 32, 215–234 (2000).
Tian, G.F. et al. An astrocytic basis of epilepsy. Nat. Med. 11, 973–981 (2005).
Takano, T. et al. Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 9, 260–267 (2006).
Kasischke, K.A., Vishwasrao, H.D., Fisher, P.J., Zipfel, W.R. & Webb, W.W. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science 305, 99–103 (2004).
Chance, B., Cohen, F., Jobsis, F. & Schoener, B. Intracellular oxidation-reduction states in vivo. Science 137, 499–508 (1962).
Hadjikhani, N. et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc. Natl. Acad. Sci. USA 98, 4687–4692 (2001).
Nedergaard, M. Mechanisms of brain damage in focal cerebral ischemia. Acta Neurol. Scand. 77, 81–101 (1988).
Mayevsky, A., Zarchin, N. & Friedli, C.M. Factors affecting the oxygen balance in the awake cerebral cortex exposed to spreading depression. Brain Res. 236, 93–105 (1982).
Mayevsky, A. & Weiss, H.R. Cerebral blood flow and oxygen consumption in cortical spreading depression. J. Cereb. Blood Flow Metab. 11, 829–836 (1991).
Mayevsky, A., Lebourdais, S. & Chance, B. The interrelation between brain PO2 and NADH oxidation-reduction state in the gerbil. J. Neurosci. Res. 5, 173–182 (1980).
Tomita, M. et al. Initial oligemia with capillary flow stop followed by hyperemia during K+-induced cortical spreading depression in rats. J. Cereb. Blood Flow Metab. 25, 742–747 (2005).
Ayata, C. et al. Pronounced hypoperfusion during spreading depression in mouse cortex. J. Cereb. Blood Flow Metab. 24, 1172–1182 (2004).
Hansen, A.J., Quistorff, B. & Gjedde, A. Relationship between local changes in cortical blood flow and extracellular K+ during spreading depression. Acta Physiol. Scand. 109, 1–6 (1980).
Fabricius, M. & Lauritzen, M. Transient hyperemia succeeds oligemia in the wake of cortical spreading depression. Brain Res. 602, 350–353 (1993).
Revsbech, N. An oxygen microsensor with a guard cathode. Limnol. Oceanogr. 34, 474–478 (1989).
Van Harreveld, A. & Fifkova, E. Glutamate release from the retina during spreading depression. J. Neurobiol. 2, 13–29 (1970).
Hansen, A.J. Effect of anoxia on ion distribution in the brain. Physiol. Rev. 65, 101–148 (1985).
Hasbani, M.J., Schlief, M.L., Fisher, D.A. & Goldberg, M.P. Dendritic spines lost during glutamate receptor activation reemerge at original sites of synaptic contact. J. Neurosci. 21, 2393–2403 (2001).
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000).
Oliva, A.A., Jr, Jiang, M., Lam, T., Smith, K.L. & Swann, J.W. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J. Neurosci. 20, 3354–3368 (2000).
Takano, T. et al. Receptor-mediated glutamate release from volume sensitive channels in astrocytes. Proc. Natl. Acad. Sci. USA 102, 16466–16471 (2005).
Capobianco, D.J. & Dodick, D.W. Diagnosis and treatment of cluster headache. Semin. Neurol. 26, 242–259 (2006).
Mayevsky, A. & Sclarksy, D.L. Correlation of brain NADH redox state, K+, PO2 and electrical activity during hypoxia, ischemia and spreading depression. Adv. Exp. Med. Biol. 159, 129–141 (1983).
Schulte, M.L. & Hudetz, A.G. Functional hyperemic response in the rat visual cortex under halothane anesthesia. Neurosci. Lett. 394, 63–68 (2006).
Hansen, A.J. & Nedergaard, M. Brain ion homeostasis in cerebral ischemia. Neurochem. Pathol. 9, 195–209 (1988).
Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat. Rev. Neurosci. 5, 347–360 (2004).
Van Harreveld, A. Changes in volume of cortical neuronal elements during asphyxiation. Am. J. Physiol. 101, 233–242 (1957).
Somjen, G.G. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol. Rev. 81, 1065–1096 (2001).
Nicholson, C., Kraig, R.P., ten Bruggencate, G., Stockle, H. & Steinberg, R. Potassium, calcium, chloride and sodium changes in extracellular space during spreading depression in cerebellum [proceedings]. Arzneimittelforschung 28, 874–875 (1978).
Nedergaard, M. & Hansen, A.J. Characterization of cortical depolarizations evoked in focal cerebral ischemia. J. Cereb. Blood Flow Metab. 13, 568–574 (1993).
Grafstein, B. Mechanism of spreading cortical depression. J. Neurophysiol. 19, 154–171 (1956).
Nedergaard, M. & Astrup, J. Infarct rim: effect of hyperglycemia on direct current potential and [14C]2-deoxyglucose phosphorylation. J. Cereb. Blood Flow Metab. 6, 607–615 (1986).
Umegaki, M. et al. Peri-infarct depolarizations reveal penumbra-like conditions in striatum. J. Neurosci. 25, 1387–1394 (2005).
Mayevsky, A. et al. Cortical spreading depression recorded from the human brain using a multiparametric monitoring system. Brain Res. 740, 268–274 (1996).
Mayevsky, A., Manor, T., Meilin, S., Doron, A. & Ouaknine, G.E. Real-time multiparametric monitoring of the injured human cerebral cortex–a new approach. Acta Neurochir. Suppl. 71, 78–81 (1998).
Fabricius, M. et al. Cortical spreading depression and peri-infarct depolarization in acutely injured human cerebral cortex. Brain 129, 778–790 (2006).
Strong, A.J. & Dardis, R. Depolarisation phenomena in traumatic and ischaemic brain injury. Adv. Tech. Stand. Neurosurg. 30, 3–49 (2005).
Gorji, A. Spreading depression: a review of the clinical relevance. Brain Res. Brain Res. Rev. 38, 33–60 (2001).
Bolay, H. et al. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat. Med. 8, 136–142 (2002).
Meyer, J.S., Thornby, J., Crawford, K. & Rauch, G.M. Reversible cognitive decline accompanies migraine and cluster headaches. Headache 40, 638–646 (2000).
Calandre, E.P., Bembibre, J., Arnedo, M.L. & Becerra, D. Cognitive disturbances and regional cerebral blood flow abnormalities in migraine patients: their relationship with the clinical manifestations of the illness. Cephalalgia 22, 291–302 (2002).
Waldie, K.E., Hausmann, M., Milne, B.J. & Poulton, R. Migraine and cognitive function: a life-course study. Neurology 59, 904–908 (2002).
Riva, D. et al. Cognitive and behavioural effects of migraine in childhood and adolescence. Cephalalgia 26, 596–603 (2006).
McKendrick, A.M. & Badcock, D.R. Decreased visual field sensitivity measured 1 day, then 1 week, after migraine. Invest. Ophthalmol. Vis. Sci. 45, 1061–1070 (2004).
Shepherd, A.J. Colour vision in migraine: selective deficits for S-cone discriminations. Cephalalgia 25, 412–423 (2005).
Akin, R., Unay, B., Sarici, S.U., Ulas, U. & Gokcay, E. Evaluation of visual evoked potentials in children with headache. Turk. J. Pediatr. 47, 150–152 (2005).
Yenice, O. et al. Assessment of spatial-contrast function and short-wavelength sensitivity deficits in patients with migraine. Eye 21, 218–223 (2007).
Ayata, C., Jin, H., Kudo, C., Dalkara, T. & Moskowitz, M.A. Suppression of cortical spreading depression in migraine prophylaxis. Ann. Neurol. 59, 652–661 (2006).
Nimmerjahn, A., Kirchhoff, F., Kerr, J. & Helmchen, F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat. Methods 1, 31–37 (2004).
Acknowledgements
This work was supported in part by grants NS30007 and NS38073 from the US National Institutes of Health and the National Institute of Neurological Disorders and Stroke (to M.N.), the New York State Spinal Cord Injury Program, The Dana Foundation and the Philip-Morris Organization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
Local cerebral blood flow change during CSD in mice anesthetized with urethane + α-chloralose, 2% isoflurane, or 50 mg kg−1 sodium pentobarbital. (PDF 181 kb)
Supplementary Video 1
Imaging of NADH fluorescence change during CSD in mouse cortex at a depth of 50 μm (same experiments as shown in Fig. 1a in pseudocolor). Time-course images were captured with 3-s interval and displayed as 3.5 frames per second. (MOV 4745 kb)
Supplementary Video 2
A small penetrating artery imaged 45 μm below the cortical surface (same as shown in Fig. 3a). The artery first dilated during the passage of a CSD wave followed by a prolonged constriction. The artery was visualized by systemic administration of FITC-dextran. Images were captured at 2-s interval and displayed as 12 frame per second. (MOV 842 kb)
Supplementary Video 3
Simultaneous imaging of NADH fluorescence and blood vessels. NADH signal is in grayscale and blood vessels are shown in green. Both arteries and veins are visible in the field. Images were taken at 50 μm from the surface, and captured at 3-s interval and displayed as 9 frame per second. (MOV 2901 kb)
Supplementary Video 4
Imaging of a YFP-expressing neuron cell body at 125 μm deep during a wave of CSD (same as shown in Fig. 5d). The cell body swelled during CSD. Images were captured at 3-s interval and displayed as 12 frame per second. (MOV 514 kb)
Supplementary Video 5
Imaging of NADH fluorescence change during CSD in mouse anesthetized with 50 mg kg−1 pentobarbital. Different anesthesia did not alter the appearance of NADH pattern. Time-course images were captured with 5-s interval and displayed as 3.5 frames per second. (MOV 1089 kb)
Rights and permissions
About this article
Cite this article
Takano, T., Tian, GF., Peng, W. et al. Cortical spreading depression causes and coincides with tissue hypoxia. Nat Neurosci 10, 754–762 (2007). https://doi.org/10.1038/nn1902
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1902
This article is cited by
-
Dynamic Measurements of Cerebral Blood Flow Responses to Cortical Spreading Depolarization in the Murine Endovascular Perforation Subarachnoid Hemorrhage Model
Translational Stroke Research (2023)
-
Neuromonitoring in Children with Cerebrovascular Disorders
Neurocritical Care (2023)
-
Alterations in metabolic flux in migraine and the translational relevance
The Journal of Headache and Pain (2022)
-
Cortical Anoxic Spreading Depolarization During Cardiac Arrest is Associated with Remote Effects on Peripheral Blood Pressure and Postresuscitation Neurological Outcome
Neurocritical Care (2022)
-
The Critical Role of Spreading Depolarizations in Early Brain Injury: Consensus and Contention
Neurocritical Care (2022)