Abstract
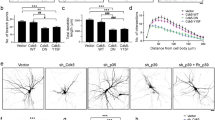
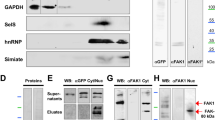
Regulated growth and arborization of dendritic processes are critical to the formation of functional neuronal networks. Here we identify β-catenin as a critical mediator of dendritic morphogenesis. We found that increasing the intracellular levels of β-catenin and other members of the cadherin/catenin complex, namely N-cadherin and αN-catenin, enhances dendritic arborization in rat hippocampal neurons, an effect that does not require Wnt/β-catenin-dependent transcription. Conversely, proteins that sequester β-catenin decreased dendritic branch tip number and total dendritic branch length. Enhancement of dendritic growth elicited by depolarization requires β-catenin and increased Wnt release. These results identify Wnt/β-catenin signaling as an important mediator of dendritic development and suggest that the intracellular level of the cadherin/catenin complex is a limiting factor during critical stages of dendritic morphogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
McAllister, A.K. Cellular and molecular mechanisms of dendrite growth. Cereb. Cortex 10, 963–973 (2000).
Cline, H.T. Dendritic arbor development and synaptogenesis. Curr. Opin. Neurobiol. 11, 118–126 (2001).
Scott, E.K. & Luo, L. How do dendrites take their shape? Nat. Neurosci. 4, 359–365 (2001).
Wong, R.O.L. & Ghosh, A. Activity-dependent regulation of dendritic growth and patterning. Nat. Rev. Neurosci. 3, 803–812 (2002).
Benson, D.L. & Tanaka, H. N-cadherin redistribution during synaptogenesis in hippocampal neurons. J. Neurosci. 18, 6892–6904 (1998).
Gumbiner, B.M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84, 345–357 (1996).
Luo, L. et al. Differential effects of the Rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature 379, 837–840 (1996).
Nakayama, A.Y., Harms, M.B. & Luo, L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J. Neurosci. 20, 5329–5338 (2000).
Li, Z., Van Aelst, L. & Cline, H.T. Rho GTPases regulate distinct aspects of dendritic arbor growth in Xenopus central neurons in vivo. Nat. Neurosci. 3, 217–225 (2000).
Wong, W.T., Faulkner-Jones, B.E., Sanes, J.R. & Wong, R.O. Rapid dendritic remodeling in the developing retina: dependence on neurotransmission and reciprocal regulation by Rac and Rho. J. Neurosci. 20, 5024–5036 (2000).
Sin, W.C., Haas, K., Ruthazer, E.S. & Cline, H.T. Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature 419, 475–480 (2002).
Cadigan, K.M. & Nusse, R. Wnt signaling: a common theme in animal development. Genes Dev. 11, 3286–3305 (1997).
Polakis, P. Wnt signaling and cancer. Genes Dev. 14, 1837–1851 (2000).
Bienz, M. & Clevers, H. Linking colorectal cancer to Wnt signaling. Cell 103, 311–320 (2000).
Chenn, A. & Walsh, C.A. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297, 365–369 (2002).
Thomas, K.R., Deng, C.X. & Capecchi, M.R. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Proc. Natl. Acad. Sci. USA 87, 7688–7692 (1990).
McMahon, A.P. & Bradley, A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell 62, 1073–1085 (1990).
Galceran, J., Miyashita-Lin, E.M., Devaney, E., Rubenstein, J.L. & Grosschedl, R. Hippocampus development and generation of dentate gyrus granule cells is regulated by LEF1. Development 127, 469–482 (2000).
Lee, S.M., Tole, S., Grove, E. & McMahon, A.P. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development 127, 457–467 (2000).
Brault, V. et al. Inactivation of the β-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253–1264 (2001).
Barth, A.I., Stewart, D.B. & Nelson, W.J. T cell factor-activated transcription is not sufficient to induce anchorage-independent growth of epithelial cells expressing mutant β-catenin. Proc. Natl. Acad. Sci. USA 96, 4947–4952 (1999).
Kintner, C. Regulation of embryonic cell adhesion by the cadherin cytoplasmic domain. Cell 69, 225–236 (1992).
Fujimori, T. & Takeichi, M. Disruption of epithelial cell-cell adhesion by exogenous expression of a mutated nonfunctional N-cadherin. Mol. Biol. Cell 4, 37–47 (1993).
Sanson, B., White, P. & Vincent, J.P. Uncoupling cadherin-based adhesion from wingless signalling in Drosophila. Nature 383, 627–630 (1996).
Sadot, E., Simcha, I., Shtutman, M., Ben-Ze'ev, A. & Geiger, B. Inhibition of β-catenin-mediated transactivation by cadherin derivatives. Proc. Natl. Acad. Sci. USA 95, 15339–15344 (1998).
Wieschaus, E. & Riggleman, R. Autonomous requirements for the segment polarity gene armadillo during Drosophila embryogenesis. Cell 49, 177–184 (1987).
Riese, J. et al. LEF-1, a nuclear factor coordinating signaling inputs from wingless and decapentaplegic. Cell 88, 777–787 (1997).
van de Wetering, M. et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88, 789–799 (1997).
Montross, W.T., Ji, H. & McCrea, P.D. A β-catenin/engrailed chimera selectively suppresses Wnt signaling. J. Cell Sci. 113, 1759–1770 (2000).
Hsu, S.C., Galceran, J. & Grosschedl, R. Modulation of transcriptional regulation by LEF-1 in response to Wnt-1 signaling and association with β-catenin. Mol. Cell Biol. 18, 4807–4818 (1998).
Giese, K., Amsterdam, A. & Grosschedl, R. DNA-binding properties of the HMG domain of the lymphoid-specific transcriptional regulator LEF-1. Genes Dev. 5, 2567–2578 (1991).
Korinek, V. et al. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 275, 1784–1787 (1997).
Redmond, L., Kashani, A.H. & Ghosh, A. Calcium regulation of dendritic growth via CaM kinase IV and CREB-mediated transcription. Neuron 34, 999–1010 (2002).
Rajan, I. & Cline, H.T. Glutamate receptor activity is required for normal development of tectal cell dendrites in vivo. J. Neurosci. 18, 7836–7846 (1998).
Rajan, I., Witte, S. & Cline, H.T. NMDA receptor activity stabilizes presynaptic retinotectal axons and postsynaptic optic tectal cell dendrites in vivo. J. Neurobiol. 38, 357–368 (1999).
Deisseroth, K., Heist, E.K. & Tsien, R.W. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature 399, 198–202 (1998).
Glinka, A. et al. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391, 357–362 (1998).
Zorn, A.M. Wnt signalling: antagonistic Dickkopfs. Curr. Biol. 11, R592–595 (2001).
Reya, T. et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423, 409–414 (2003).
Banker, G. & Goslin, K. (eds.) Culturing Nerve Cells (MIT Press, Cambridge, Massachusetts, 1998).
Hsieh, J.C., Rattner, A., Smallwood, P.M. & Nathans, J. Biochemical characterization of Wnt-frizzled interactions using a soluble, biologically active vertebrate Wnt protein. Proc. Natl. Acad. Sci. USA 96, 3546–3571 (1999).
Togashi, H. et al. Cadherin regulates dendritic spine morphogenesis. Neuron 35, 77–89 (2002).
Roura, S. et al. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation Independent regulation of adherens and tight junctions by tyrosine phosphorylation in Caco-2 cells. J. Biol. Chem. 274, 36734–36740 (1999).
Murase, S., Mosser, E. & Schuman, E.M. Depolarization drives β-Catenin into neuronal spines promoting changes in synaptic structure and function. Neuron 35, 91–105 (2002).
Maretto, S. et al. Mapping Wnt/β-catenin signaling during mouse development and in colorectal tumors. Proc. Natl. Acad. Sci. USA 100, 3299–3304 (1993).
Uchida, N., Honjo, Y., Johnson, K.R., Wheelock, M.J. & Takeichi, M. The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J. Cell Biol. 135, 767–779 (1996).
Hall, A.C., Lucas, F.R. & Salinas, P.C. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell 100, 525–535 (2000).
Packard, M. et al. The Drosophila Wnt, Wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell 111, 319–330 (2002).
Xia, Z., Dudek, H., Miranti, C.K. & Greenberg, M.E. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J. Neurosci. 16, 5425–5436 (1996).
Acknowledgements
We thank A. Barth, W. J. Nelson, S. Bamji, L. Reichardt, D. Benson, D. Colman, S. Aaronson, T. Chatila, H. Clevers, R. Grosschedl, L. Luo, P. McCrea, M. Waterman, and L. Ailles for DNA constructs, K. Deisseroth and S. Singla for the Wnt conditioned medium, and N. Calakos for the astryocyte cultures. We thank T. Lizama, L. Esaura and J. Fisher for hippocampal culture preparation and excellent technical assistance. We are grateful to C. Garner, L. Luo, K. Deisseroth and S. Singla for comments on the manuscript and to members of the Malenka lab for discussion. This work was supported by grants from the National Institutes of Health (to R.C.M) and a Wellcome International Prize Travelling Research Fellowship (to X.Y.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Yu, X., Malenka, R. β-catenin is critical for dendritic morphogenesis. Nat Neurosci 6, 1169–1177 (2003). https://doi.org/10.1038/nn1132
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1132
This article is cited by
-
Proteomics of the dentate gyrus reveals semantic dementia specific molecular pathology
Acta Neuropathologica Communications (2022)
-
Simvastatin rescues memory and granule cell maturation through the Wnt/β-catenin signaling pathway in a mouse model of Alzheimer’s disease
Cell Death & Disease (2022)
-
KIF5C deficiency causes abnormal cortical neuronal migration, dendritic branching, and spine morphology in mice
Pediatric Research (2022)
-
Characterization of genome-wide association study data reveals spatiotemporal heterogeneity of mental disorders
BMC Medical Genomics (2020)
-
Targeting β-Catenin in GLAST-Expressing Cells: Impact on Anxiety and Depression-Related Behavior and Hippocampal Proliferation
Molecular Neurobiology (2019)