Abstract
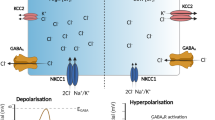
KCC2 is a neuron-specific K+-Cl− co-transporter that maintains a low intracellular Cl− concentration that is essential for hyperpolarizing inhibition mediated by GABAA receptors. Deficits in KCC2 activity occur in disease states associated with pathophysiological glutamate release. However, the mechanisms by which elevated glutamate alters KCC2 function are unknown. The phosphorylation of KCC2 residue Ser940 is known to regulate its surface activity. We found that NMDA receptor activity and Ca2+ influx caused the dephosphorylation of Ser940 in dissociated rat neurons, leading to a loss of KCC2 function that lasted longer than 20 min. Protein phosphatase 1 mediated the dephosphorylation events of Ser940 that coincided with a deficit in hyperpolarizing GABAergic inhibition resulting from the loss of KCC2 activity. Blocking dephosphorylation of Ser940 reduced the glutamate-induced downregulation of KCC2 and substantially improved the maintenance of hyperpolarizing GABAergic inhibition. Reducing the downregulation of KCC2 therefore has therapeutic potential in the treatment of neurological disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Payne, J.A., Stevenson, T.J. & Donaldson, L.F. Molecular characterization of a putative K-Cl cotransporter in rat brain. A neuronal-specific isoform. J. Biol. Chem. 271, 16245–16252 (1996).
Rivera, C. et al. The K+/Cl– co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397, 251–255 (1999).
Hübner, C.A. et al. Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron 30, 515–524 (2001).
Ben-Ari, Y. Excitatory actions of GABA during development: the nature of the nurture. Nat. Rev. Neurosci. 3, 728–739 (2002).
Lu, J., Karadsheh, M. & Delpire, E. Developmental regulation of the neuronal-specific isoform of K-Cl cotransporter KCC2 in postnatal rat brains. J. Neurobiol. 39, 558–568 (1999).
Blaesse, P., Airaksinen, M.S., Rivera, C. & Kaila, K. Cation-chloride cotransporters and neuronal function. Neuron 61, 820–838 (2009).
Kahle, K.T. et al. Roles of the cation-chloride cotransporters in neurological disease. Nat. Clin. Pract. Neurol. 4, 490–503 (2008).
Coull, J.A. et al. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424, 938–942 (2003).
Cramer, S.W. et al. The role of cation-dependent chloride transporters in neuropathic pain following spinal cord injury. Mol. Pain 4, 36 (2008).
Lu, Y., Zheng, J., Xiong, L., Zimmermann, M. & Yang, J. Spinal cord injury-induced attenuation of GABAergic inhibition in spinal dorsal horn circuits is associated with down-regulation of the chloride transporter KCC2 in rat. J. Physiol. (Lond.) 586, 5701–5715 (2008).
Price, T.J., Cervero, F., Gold, M.S., Hammond, D.L. & Prescott, S.A. Chloride regulation in the pain pathway. Brain Res. Rev. 60, 149–170 (2009).
Wu, L.A., Huang, J., Wang, W., Wang, X.J. & Wu, S.X. Down-regulation of K+-Cl− co-transporter 2 in mouse medullary dorsal horn contributes to the formalin-induced inflammatory orofacial pain. Neurosci. Lett. 457, 36–40 (2009).
Galeffi, F., Sah, R., Pond, B.B., George, A. & Schwartz-Bloom, R.D. Changes in intracellular chloride after oxygen-glucose deprivation of the adult hippocampal slice: effect of diazepam. J. Neurosci. 24, 4478–4488 (2004).
Jaenisch, N., Witte, O.W. & Frahm, C. Downregulation of potassium chloride cotransporter KCC2 after transient focal cerebral ischemia. Stroke 41, e151–e159 (2010).
Papp, E., Rivera, C., Kaila, K. & Freund, T.F. Relationship between neuronal vulnerability and potassium-chloride cotransporter 2 immunoreactivity in hippocampus following transient forebrain ischemia. Neuroscience 154, 677–689 (2008).
Ginsberg, M.D. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology 55, 363–389 (2008).
Kristensen, B.W., Noraberg, J. & Zimmer, J. The GABAA receptor agonist THIP is neuroprotective in organotypic hippocampal slice cultures. Brain Res. 973, 303–306 (2003).
Clarkson, A.N., Huang, B.S., MacIsaac, S.E., Mody, I. & Carmichael, S.T. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature 468, 305–309 (2010).
Jin, X., Huguenard, J.R. & Prince, D.A. Impaired Cl– extrusion in layer V pyramidal neurons of chronically injured epileptogenic neocortex. J. Neurophysiol. 93, 2117–2126 (2005).
Pathak, H.R. et al. Disrupted dentate granule cell chloride regulation enhances synaptic excitability during development of temporal lobe epilepsy. J. Neurosci. 27, 14012–14022 (2007).
Rivera, C. et al. Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. J. Neurosci. 24, 4683–4691 (2004).
Cohen, I., Navarro, V., Clemenceau, S., Baulac, M. & Miles, R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science 298, 1418–1421 (2002).
Huberfeld, G. et al. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J. Neurosci. 27, 9866–9873 (2007).
Semah, F. et al. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology 51, 1256–1262 (1998).
Stephen, L.J., Kwan, P. & Brodie, M.J. Does the cause of localisation-related epilepsy influence the response to antiepileptic drug treatment? Epilepsia 42, 357–362 (2001).
Kahle, K.T. et al. WNK3 modulates transport of Cl– in and out of cells: implications for control of cell volume and neuronal excitability. Proc. Natl. Acad. Sci. USA 102, 16783–16788 (2005).
Rinehart, J. et al. Sites of regulated phosphorylation that control K-Cl cotransporter activity. Cell 138, 525–536 (2009).
Watanabe, M., Wake, H., Moorhouse, A.J. & Nabekura, J. Clustering of neuronal K+-Cl− cotransporters in lipid rafts by tyrosine phosphorylation. J. Biol. Chem. 284, 27980–27988 (2009).
Wake, H. et al. Early changes in KCC2 phosphorylation in response to neuronal stress result in functional downregulation. J. Neurosci. 27, 1642–1650 (2007).
Lee, H.H., Jurd, R. & Moss, S.J. Tyrosine phosphorylation regulates the membrane trafficking of the potassium chloride co-transporter KCC2. Mol. Cell. Neurosci. 45, 173–179 (2010).
Lee, H.H. et al. Direct protein kinase C-dependent phosphorylation regulates the cell surface stability and activity of the potassium chloride cotransporter KCC2. J. Biol. Chem. 282, 29777–29784 (2007).
During, M.J. & Spencer, D.D. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet 341, 1607–1610 (1993).
Kitamura, A. et al. Sustained depolarizing shift of the GABA reversal potential by glutamate receptor activation in hippocampal neurons. Neurosci. Res. 62, 270–277 (2008).
Banke, T.G. & McBain, C.J. GABAergic input onto CA3 hippocampal interneurons remains shunting throughout development. J. Neurosci. 26, 11720–11725 (2006).
Hewitt, S.A., Wamsteeker, J.I., Kurz, E.U. & Bains, J.S. Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nat. Neurosci. 12, 438–443 (2009).
Vargová, L., Jendelová, P., Chvátal, A. & Syková, E. Glutamate, NMDA, and AMPA induced changes in extracellular space volume and tortuosity in the rat spinal cord. J. Cereb. Blood Flow Metab. 21, 1077–1089 (2001).
Plotkin, M.D., Snyder, E.Y., Hebert, S.C. & Delpire, E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA′s excitatory role in immature brain. J. Neurobiol. 33, 781–795 (1997).
Cohen, P. The structure and regulation of protein phosphatases. Annu. Rev. Biochem. 58, 453–508 (1989).
Földy, C., Lee, S.H., Morgan, R.J. & Soltesz, I. Regulation of fast-spiking basket cell synapses by the chloride channel ClC-2. Nat. Neurosci. 13, 1047–1049 (2010).
Staley, K., Smith, R., Schaack, J., Wilcox, C. & Jentsch, T.J. Alteration of GABAA receptor function following gene transfer of the CLC-2 chloride channel. Neuron 17, 543–551 (1996).
Thompson, S.M. & Gahwiler, B.H. Activity-dependent disinhibition. II. Effects of extracellular potassium, furosemide, and membrane potential on ECl– in hippocampal CA3 neurons. J. Neurophysiol. 61, 512–523 (1989).
Banke, T.G. & Gegelashvili, G. Tonic activation of group I mGluRs modulates inhibitory synaptic strength by regulating KCC2 activity. J. Physiol. (Lond.) 586, 4925–4934 (2008).
Nabekura, J. et al. Reduction of KCC2 expression and GABAA receptor–mediated excitation after in vivo axonal injury. J. Neurosci. 22, 4412–4417 (2002).
Toyoda, H. et al. Induction of NMDA and GABAA receptor–mediated Ca2+ oscillations with KCC2 mRNA downregulation in injured facial motoneurons. J. Neurophysiol. 89, 1353–1362 (2003).
Bladin, C.F. et al. Seizures after stroke: a prospective multicenter study. Arch. Neurol. 57, 1617–1622 (2000).
Garrett, M.C. et al. Predictors of seizure onset after intracerebral hemorrhage and the role of long-term antiepileptic therapy. J. Crit. Care 24, 335–339 (2009).
Cavus, I. et al. Decreased hippocampal volume on MRI is associated with increased extracellular glutamate in epilepsy patients. Epilepsia 49, 1358–1366 (2008).
Palma, E. et al. Anomalous levels of Cl− transporters in the hippocampal subiculum from temporal lobe epilepsy patients make GABA excitatory. Proc. Natl. Acad. Sci. USA 103, 8465–8468 (2006).
Rivera, C. et al. BDNF-induced TrkB activation downregulates the K+-Cl− cotransporter KCC2 and impairs neuronal Cl− extrusion. J. Cell Biol. 159, 747–752 (2002).
Acknowledgements
We thank Y. Haydon and L.K. Chau for critical comments on the manuscript and H. Tang and L. Silayeva for providing technical assistance. The work was supported in part by US National Institutes of Health/National Institute of Neurological Disorders and Stroke grants NS036296, NS047478, NS048045 and NS054900. H.H.C.L. is supported by a Postdoctoral Fellowship from the American Heart Association.
Author information
Authors and Affiliations
Contributions
H.H.C.L. and J.A.W. performed biochemical experiments, and T.Z.D. and P.A.D. performed electrophysiological recordings. H.H.C.L., T.Z.D. and S.J.M. designed the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 (PDF 669 kb)
Rights and permissions
About this article
Cite this article
Lee, H., Deeb, T., Walker, J. et al. NMDA receptor activity downregulates KCC2 resulting in depolarizing GABAA receptor–mediated currents. Nat Neurosci 14, 736–743 (2011). https://doi.org/10.1038/nn.2806
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2806
This article is cited by
-
Neuronal K+-Cl- cotransporter KCC2 as a promising drug target for epilepsy treatment
Acta Pharmacologica Sinica (2024)
-
The Coordinating Role of the Actin Cytoskeleton in Short-Term Neural Network Plasticity Involving Excitatory and Inhibitory Synapses
Neuroscience and Behavioral Physiology (2024)
-
Emergence of consciousness from anesthesia through ubiquitin degradation of KCC2 in the ventral posteromedial nucleus of the thalamus
Nature Neuroscience (2023)
-
Preservation of KCC2 expression in axotomized abducens motoneurons and its enhancement by VEGF
Brain Structure and Function (2023)
-
Why won’t it stop? The dynamics of benzodiazepine resistance in status epilepticus
Nature Reviews Neurology (2022)