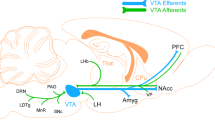
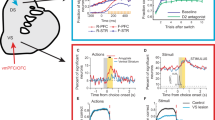
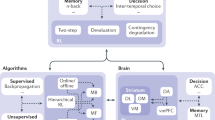
Abstract
Over the last decade and a half, reinforcement learning models have fostered an increasingly sophisticated understanding of the functions of dopamine and cortico-basal ganglia-thalamo-cortical (CBGTC) circuits. More recently, these models, and the insights that they afford, have started to be used to understand important aspects of several psychiatric and neurological disorders that involve disturbances of the dopaminergic system and CBGTC circuits. We review this approach and its existing and potential applications to Parkinson's disease, Tourette's syndrome, attention-deficit/hyperactivity disorder, addiction, schizophrenia and preclinical animal models used to screen new antipsychotic drugs. The approach's proven explanatory and predictive power bodes well for the continued growth of computational psychiatry and computational neurology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Charney, D.S. et al. Neuroscience research agenda to guide development of a pathophysiologically based classification system. in A Research Agenda for DSM-V (eds. Kupfer, D.J., First, M.B. & Regier, D.A.) 31–83 (American Psychiatric Association, Washington, D.C., 2002).
Hyman, S.E. Can neuroscience be integrated into the DSM-V? Nat. Rev. Neurosci. 8, 725–732 (2007).
Cools, R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease. Neurosci. Biobehav. Rev. 30, 1–23 (2006).
Frank, M.J., Samanta, J., Moustafa, A.A. & Sherman, S.J. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science 318, 1309–1312 (2007).
Maia, T.V. Reinforcement learning, conditioning, and the brain: successes and challenges. Cogn. Affect. Behav. Neurosci. 9, 343–364 (2009).
Dayan, P., Niv, Y., Seymour, B. & Daw, N.D. The misbehavior of value and the discipline of the will. Neural Netw. 19, 1153–1160 (2006).
McClure, S.M., Daw, N.D. & Montague, P.R. A computational substrate for incentive salience. Trends Neurosci. 26, 423–428 (2003).
Dayan, P. & Balleine, B.W. Reward, motivation, and reinforcement learning. Neuron 36, 285–298 (2002).
Niv, Y., Daw, N.D., Joel, D. & Dayan, P. Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology (Berl.) 191, 507–520 (2007).
Wiecki, T.V. & Frank, M.J. Neurocomputational models of motor and cognitive deficits in Parkinson's disease. Prog. Brain Res. 183, 275–297 (2010).
Frank, M.J., Santamaria, A., O'Reilly, R.C. & Willcutt, E. Testing computational models of dopamine and noradrenaline dysfunction in attention deficit/hyperactivity disorder. Neuropsychopharmacology 32, 1583–1599 (2007).
Frank, M.J., Scheres, A. & Sherman, S.J. Understanding decision-making deficits in neurological conditions: insights from models of natural action selection. Phil. Trans. R. Soc. Lond. B 362, 1641–1654 (2007).
Waltz, J.A., Frank, M.J., Robinson, B.M. & Gold, J.M. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol. Psychiatry 62, 756–764 (2007).
Redish, A.D. Addiction as a computational process gone awry. Science 306, 1944–1947 (2004).
Sutton, R.S. & Barto, A.G. Reinforcement Learning: An Introduction (MIT Press, Cambridge, Massachusetts, 1998).
Bayer, H.M. & Glimcher, P.W. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron 47, 129–141 (2005).
Bayer, H.M., Lau, B. & Glimcher, P.W. Statistics of midbrain dopamine neuron spike trains in the awake primate. J. Neurophysiol. 98, 1428–1439 (2007).
Daw, N.D., Kakade, S. & Dayan, P. Opponent interactions between serotonin and dopamine. Neural Netw. 15, 603–616 (2002).
Schultz, W. Dopamine signals for reward value and risk: basic and recent data. Behav. Brain Funct. 6, 24 (2010).
Morris, G., Schmidt, R. & Bergman, H. Striatal action-learning based on dopamine concentration. Exp. Brain Res. 200, 307–317 (2010).
Tsai, H.C. et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324, 1080–1084 (2009).
Maia, T.V. Two-factor theory, the actor-critic model, and conditioned avoidance. Learn. Behav. 38, 50–67 (2010).
O'Doherty, J. et al. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science 304, 452–454 (2004).
Alexander, G.E., DeLong, M.R. & Strick, P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 9, 357–381 (1986).
Yin, H.H. & Knowlton, B.J. The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 7, 464–476 (2006).
Mink, J.W. The basal ganglia: focused selection and inhibition of competing motor programs. Prog. Neurobiol. 50, 381–425 (1996).
Prescott, T.J., Gurney, K. & Redgrave, P. Basal ganglia. in The Handbook of Brain Theory and Neural Networks (ed. Arbib, M.A.) 147–151 (MIT Press, Cambridge, Massachusetts, 2003).
Frank, M.J. Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and non-medicated Parkinsonism. J. Cogn. Neurosci. 17, 51–72 (2005).
Samejima, K., Ueda, Y., Doya, K. & Kimura, M. Representation of action-specific reward values in the striatum. Science 310, 1337–1340 (2005).
Albin, R.L., Young, A.B. & Penney, J.B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–375 (1989).
DeLong, M.R. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 13, 281–285 (1990).
Nambu, A., Tokuno, H. & Takada, M. Functional significance of the cortico-subthalamo-pallidal 'hyperdirect' pathway. Neurosci. Res. 43, 111–117 (2002).
Bogacz, R. & Gurney, K. The basal ganglia and cortex implement optimal decision making between alternative actions. Neural Comput. 19, 442–477 (2007).
Brown, J.W., Bullock, D. & Grossberg, S. How laminar frontal cortex and basal ganglia circuits interact to control planned and reactive saccades. Neural Netw. 17, 471–510 (2004).
Frank, M.J. Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making. Neural Netw. 19, 1120–1136 (2006).
Gurney, K., Prescott, T.J. & Redgrave, P. A computational model of action selection in the basal ganglia. II. Analysis and simulation of behaviour. Biol. Cybern. 84, 411–423 (2001).
Frank, M.J. & Fossella, J.A. Neurogenetics and pharmacology of learning, motivation, and cognition. Neuropsychopharmacology 36, 133–152 (2010).
Hikida, T., Kimura, K., Wada, N., Funabiki, K. & Nakanishi, S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron 66, 896–907 (2010).
Kravitz, A.V. et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466, 622–626 (2010).
Gerfen, C.R. Molecular effects of dopamine on striatal-projection pathways. Trends Neurosci. 23, S64–S70 (2000).
Shen, W., Flajolet, M., Greengard, P. & Surmeier, D.J. Dichotomous dopaminergic control of striatal synaptic plasticity. Science 321, 848–851 (2008).
Creese, I., Sibley, D.R., Hamblin, M.W. & Leff, S.E. The classification of dopamine receptors: relationship to radioligand binding. Annu. Rev. Neurosci. 6, 43–71 (1983).
Waltz, J.A., Frank, M.J., Wiecki, T.V. & Gold, J.M. Altered probabilistic learning and response biases in schizophrenia: behavioral evidence and neurocomputational modeling. Neuropsychology published online, doi:10.1037/a0020882 (22 November 2010).
Haber, S.N. The primate basal ganglia: parallel and integrative networks. J. Chem. Neuroanat. 26, 317–330 (2003).
Postuma, R.B. & Dagher, A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb. Cortex 16, 1508–1521 (2006).
Frank, M.J., Loughry, B. & O'Reilly, R.C. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn. Affect. Behav. Neurosci. 1, 137–160 (2001).
O'Reilly, R.C. & Frank, M.J. Making working memory work: a computational model of learning in the prefrontal cortex and basal ganglia. Neural Comput. 18, 283–328 (2006).
Baier, B. et al. Keeping memory clear and stable: the contribution of human basal ganglia and prefrontal cortex to working memory. J. Neurosci. 30, 9788–9792 (2010).
Tzschentke, T.M. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog. Neurobiol. 56, 613–672 (1998).
Lobo, M.K. et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 330, 385–390 (2010).
Frank, M.J. & Claus, E.D. Anatomy of a decision: striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychol. Rev. 113, 300–326 (2006).
Kalanithi, P.S. et al. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc. Natl. Acad. Sci. USA 102, 13307–13312 (2005).
Biederman, J. & Spencer, T. Attention-deficit/hyperactivity disorder (ADHD) as a noradrenergic disorder. Biol. Psychiatry 46, 1234–1242 (1999).
Guillin, O., Abi-Dargham, A. & Laruelle, M. Neurobiology of dopamine in schizophrenia. Int. Rev. Neurobiol. 78, 1–39 (2007).
Nikolaus, S., Antke, C. & Muller, H.W. In vivo imaging of synaptic function in the central nervous system. II. Mental and affective disorders. Behav. Brain Res. 204, 32–66 (2009).
Stahl, S.M. Beyond the dopamine hypothesis to the NMDA glutamate receptor hypofunction hypothesis of schizophrenia. CNS Spectr. 12, 265–268 (2007).
Rolls, E.T., Loh, M., Deco, G. & Winterer, G. Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nat. Rev. Neurosci. 9, 696–709 (2008).
Frank, M.J., Seeberger, L.C. & O'Reilly, R.C. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science 306, 1940–1943 (2004).
Moustafa, A.A., Sherman, S.J. & Frank, M.J. A dopaminergic basis for working memory, learning and attentional shifting in Parkinsonism. Neuropsychologia 46, 3144–3156 (2008).
Cools, R., Miyakawa, A., Sheridan, M. & D'Esposito, M. Enhanced frontal function in Parkinson's disease. Brain 133, 225–233 (2010).
Mink, J.W. Basal ganglia dysfunction in Tourette's syndrome: a new hypothesis. Pediatr. Neurol. 25, 190–198 (2001).
Delfs, J.M. & Kelley, A.E. The role of D1 and D2 dopamine receptors in oral stereotypy induced by dopaminergic stimulation of the ventrolateral striatum. Neuroscience 39, 59–67 (1990).
Walters, J.R., Bergstrom, D.A., Carlson, J.H., Chase, T.N. & Braun, A.R. D1 dopamine receptor activation required for postsynaptic expression of D2 agonist effects. Science 236, 719–722 (1987).
Steeves, T.D. & Fox, S.H. Neurobiological basis of serotonin-dopamine antagonists in the treatment of Gilles de la Tourette syndrome. Prog. Brain Res. 172, 495–513 (2008).
Palminteri, S. et al. Pharmacological modulation of subliminal learning in Parkinson's and Tourette's syndromes. Proc. Natl. Acad. Sci. USA 106, 19179–19184 (2009).
Peterson, B.S. et al. Neuroanatomical circuitry. in Tourette's Syndrome: Tics, Obsessions, Compulsions. Developmental Psychopathology and Clinical Care (eds. Leckman, J.F. & Cohen, D.J.) 230–259 (John Wiley & Sons, New York, 1999).
Bohlhalter, S. et al. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain 129, 2029–2037 (2006).
Barkley, R.A. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol. Bull. 121, 65–94 (1997).
Sagvolden, T. & Sergeant, J.A. Attention deficit/hyperactivity disorder—from brain dysfunctions to behaviour. Behav. Brain Res. 94, 1–10 (1998).
Sonuga-Barke, E.J. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol. Psychiatry 57, 1231–1238 (2005).
Sagvolden, T., Johansen, E.B., Aase, H. & Russell, V.A. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav. Brain Sci. 28, 397–419 (2005).
Smith, A.J., Becker, S. & Kapur, S. A computational model of the functional role of the ventral-striatal D2 receptor in the expression of previously acquired behaviors. Neural Comput. 17, 361–395 (2005).
Pattij, T. & Vanderschuren, L.J. The neuropharmacology of impulsive behaviour. Trends Pharmacol. Sci. 29, 192–199 (2008).
Winstanley, C.A., Theobald, D.E., Dalley, J.W. & Robbins, T.W. Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacology 30, 669–682 (2005).
Kheramin, S. et al. Effects of orbital prefrontal cortex dopamine depletion on inter-temporal choice: a quantitative analysis. Psychopharmacology (Berl.) 175, 206–214 (2004).
Durstewitz, D., Seamans, J.K. & Sejnowski, T.J. Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. J. Neurophysiol. 83, 1733–1750 (2000).
Maia, T.V. & Cleeremans, A. Consciousness: converging insights from connectionist modeling and neuroscience. Trends Cogn. Sci. 9, 397–404 (2005).
Figner, B. et al. Lateral prefrontal cortex and self-control in intertemporal choice. Nat. Neurosci. 13, 538–539 (2010).
Volkow, N.D., Fowler, J.S., Wang, G.J., Swanson, J.M. & Telang, F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch. Neurol. 64, 1575–1579 (2007).
Panlilio, L.V., Thorndike, E.B. & Schindler, C.W. Blocking of conditioning to a cocaine-paired stimulus: testing the hypothesis that cocaine perpetually produces a signal of larger-than-expected reward. Pharmacol. Biochem. Behav. 86, 774–777 (2007).
Dezfouli, A. et al. A neurocomputational model for cocaine addiction. Neural Comput. 21, 2869–2893 (2009).
Redish, A.D., Jensen, S. & Johnson, A. A unified framework for addiction: vulnerabilities in the decision process. Behav. Brain Sci. 31, 415–437, discussion 437–487 (2008).
Schoenbaum, G., Roesch, M.R. & Stalnaker, T.A. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 29, 116–124 (2006).
Berridge, K.C. & Robinson, T.E. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Brain Res. Rev. 28, 309–369 (1998).
Kapur, S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry 160, 13–23 (2003).
Corlett, P.R., Honey, G.D. & Fletcher, P.C. From prediction error to psychosis: ketamine as a pharmacological model of delusions. J. Psychopharmacol. 21, 238–252 (2007).
Corlett, P.R. et al. Disrupted prediction-error signal in psychosis: evidence for an associative account of delusions. Brain 130, 2387–2400 (2007).
Murray, G.K. et al. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol. Psychiatry 13, 267–276 (2008).
Waltz, J.A. et al. Patients with schizophrenia have a reduced neural response to both unpredictable and predictable primary reinforcers. Neuropsychopharmacology 34, 1567–1577 (2009).
Strauss, G.P. et al. Deficits in positive reinforcement learning and uncertainty-driven exploration are associated with distinct aspects of negative symptoms in schizophrenia. Biol. Psychiatry (in the press).
Wadenberg, M.L. & Hicks, P.B. The conditioned avoidance response test re-evaluated: is it a sensitive test for the detection of potentially atypical antipsychotics? Neurosci. Biobehav. Rev. 23, 851–862 (1999).
Fibiger, H.C., Phillips, A.G. & Zis, A.P. Deficits in instrumental responding after 6-hydroxydopamine lesions of the nigro-neostriatal dopaminergic projection. Pharmacol. Biochem. Behav. 2, 87–96 (1974).
Fantin, G. & Bottecchia, D. Effect of nucleus accumbens destruction in rat. Experientia 40, 573–575 (1984).
Wadenberg, M.L., Ericson, E., Magnusson, O. & Ahlenius, S. Suppression of conditioned avoidance behavior by the local application of (–)sulpiride into the ventral, but not the dorsal, striatum of the rat. Biol. Psychiatry 28, 297–307 (1990).
Salamone, J.D. & Correa, M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav. Brain Res. 137, 3–25 (2002).
Yin, H.H., Ostlund, S.B. & Balleine, B.W. Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks. Eur. J. Neurosci. 28, 1437–1448 (2008).
Maia, T.V., Cooney, R.E. & Peterson, B.S. The neural bases of obsessive-compulsive disorder in children and adults. Dev. Psychopathol. 20, 1251–1283 (2008).
Dayan, P., Kakade, S. & Montague, P.R. Learning and selective attention. Nat. Neurosci. 3 Suppl: 1218–1223 (2000).
Gershman, S.J., Blei, D.M. & Niv, Y. Context, learning, and extinction. Psychol. Rev. 117, 197–209 (2010).
Maia, T.V. Fear conditioning and social groups: statistics, not genetics. Cogn. Sci. 33, 1232–1251 (2009).
Acknowledgements
Preparation of this article was funded by a Research Associate Award from the New York State Psychiatric Institute and the Research Foundation for Mental Hygiene, by National Institute of Mental Health grant R01 MH080066 and by a grant from the Michael J. Fox Foundation for Parkinson's Research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Maia, T., Frank, M. From reinforcement learning models to psychiatric and neurological disorders. Nat Neurosci 14, 154–162 (2011). https://doi.org/10.1038/nn.2723
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2723
This article is cited by
-
A neural mechanism for conserved value computations integrating information and rewards
Nature Neuroscience (2024)
-
Computational Modelling for Alcohol Use Disorder
Erkenntnis (2024)
-
Out of control: computational dynamic control dysfunction in stress- and anxiety-related disorders
Discover Mental Health (2024)
-
Reinforcement learning establishes a minimal metacognitive process to monitor and control motor learning performance
Nature Communications (2023)
-
Humans display interindividual differences in the latent mechanisms underlying fear generalization behaviour
Communications Psychology (2023)