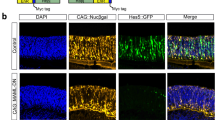
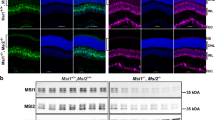
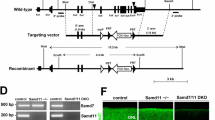
Abstract
Selective expression of retinal cone opsin genes is essential for color vision, but the mechanism mediating this process is poorly understood. Both vertebrate rod and medium wavelength–sensitive (M) cone photoreceptors differentiate by repression of a short wavelength–sensitive (S) cone differentiation program. We found that Pias3 acts in mouse cone photoreceptors to activate expression of M opsin and repress expression of S opsin, with the transcription factors Trβ2 and Rxrγ mediating preferential expression of Pias3 in M cones. Finally, we observed that Pias3 directly regulated M and S cone opsin expression by modulating the cone-enriched transcription factors Rxrγ, Rorα and Trβ1. Our results indicate that Pias3-dependent SUMOylation of photoreceptor-specific transcription factors is a common mechanism that controls both rod and cone photoreceptor subtype specification, regulating distinct molecular targets in the two cell types.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Solomon, S.G. & Lennie, P. The machinery of colour vision. Nat. Rev. Neurosci. 8, 276–286 (2007).
Osorio, D. & Vorobyev, M. A review of the evolution of animal colour vision and visual communication signals. Vision Res. 48, 2042–2051 (2008).
Collin, S.P. & Trezise, A.E. The origins of colour vision in vertebrates. Clin. Exp. Optom. 87, 217–223 (2004).
Pichaud, F., Briscoe, A. & Desplan, C. Evolution of color vision. Curr. Opin. Neurobiol. 9, 622–627 (1999).
Peichl, L. Diversity of mammalian photoreceptor properties: adaptations to habitat and lifestyle? Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 287, 1001–1012 (2005).
Röhlich, P., van Veen, T. & Szel, A. Two different visual pigments in one retinal cone cell. Neuron 13, 1159–1166 (1994).
Lukáts, A., Szabo, A., Rohlich, P., Vigh, B. & Szel, A. Photopigment coexpression in mammals: comparative and developmental aspects. Histol. Histopathol. 20, 551–574 (2005).
Applebury, M.L. et al. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron 27, 513–523 (2000).
Furukawa, T., Morrow, E.M. & Cepko, C.L. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell 91, 531–541 (1997).
Nishida, A. et al. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat. Neurosci. 6, 1255–1263 (2003).
Akhmedov, N.B. et al. A deletion in a photoreceptor-specific nuclear receptor mRNA causes retinal degeneration in the rd7 mouse. Proc. Natl. Acad. Sci. USA 97, 5551–5556 (2000).
Haider, N.B. et al. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat. Genet. 24, 127–131 (2000).
Mears, A.J. et al. Nrl is required for rod photoreceptor development. Nat. Genet. 29, 447–452 (2001).
Peng, G.H., Ahmad, O., Ahmad, F., Liu, J. & Chen, S. The photoreceptor-specific nuclear receptor Nr2e3 interacts with Crx and exerts opposing effects on the transcription of rod versus cone genes. Hum. Mol. Genet. 14, 747–764 (2005).
Ng, L., Ma, M., Curran, T. & Forrest, D. Developmental expression of thyroid hormone receptor beta2 protein in cone photoreceptors in the mouse. Neuroreport 20, 627–631 (2009).
Ng, L. et al. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat. Genet. 27, 94–98 (2001).
Roberts, M.R., Hendrickson, A., McGuire, C.R. & Reh, T.A. Retinoid X receptor (gamma) is necessary to establish the S-opsin gradient in cone photoreceptors of the developing mouse retina. Invest. Ophthalmol. Vis. Sci. 46, 2897–2904 (2005).
Roberts, M.R., Srinivas, M., Forrest, D., Morreale de Escobar, G. & Reh, T.A. Making the gradient: thyroid hormone regulates cone opsin expression in the developing mouse retina. Proc. Natl. Acad. Sci. USA 103, 6218–6223 (2006).
Applebury, M.L. et al. Transient expression of thyroid hormone nuclear receptor TRbeta2 sets S opsin patterning during cone photoreceptor genesis. Dev. Dyn. 236, 1203–1212 (2007).
Pessôa, C.N. et al. Thyroid hormone action is required for normal cone opsin expression during mouse retinal development. Invest. Ophthalmol. Vis. Sci. 49, 2039–2045 (2008).
Hennig, A.K., Peng, G.H. & Chen, S. Regulation of photoreceptor gene expression by Crx-associated transcription factor network. Brain Res. 1192, 114–133 (2008).
Onishi, A. et al. Pias3-dependent SUMOylation directs rod photoreceptor development. Neuron 61, 234–246 (2009).
Long, J., Wang, G., Matsuura, I., He, D. & Liu, F. Activation of Smad transcriptional activity by protein inhibitor of activated STAT3 (PIAS3). Proc. Natl. Acad. Sci. USA 101, 99–104 (2004).
Boggio, R., Colombo, R., Hay, R.T., Draetta, G.F. & Chiocca, S. A mechanism for inhibiting the SUMO pathway. Mol. Cell 16, 549–561 (2004).
Kliewer, S.A., Umesono, K., Mangelsdorf, D.J. & Evans, R.M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signaling. Nature 355, 446–449 (1992).
Li, D., Li, T., Wang, F., Tian, H. & Samuels, H.H. Functional evidence for retinoid X receptor (RXR) as a nonsilent partner in the thyroid hormone receptor/RXR heterodimer. Mol. Cell. Biol. 22, 5782–5792 (2002).
McCaffrery, P., Posch, K.C., Napoli, J.L., Gudas, L. & Drager, U.C. Changing patterns of the retinoic acid system in the developing retina. Dev. Biol. 158, 390–399 (1993).
Fujieda, H., Bremner, R., Mears, A.J. & Sasaki, H. Retinoic acid receptor–related orphan receptor alpha regulates a subset of cone genes during mouse retinal development. J. Neurochem. 108, 91–101 (2009).
Hwang, E.J. et al. SUMOylation of RORalpha potentiates transcriptional activation function. Biochem. Biophys. Res. Commun. 378, 513–517 (2009).
Szél, A., Rohlich, P., Mieziewska, K., Aguirre, G. & van Veen, T. Spatial and temporal differences between the expression of short- and middle-wave sensitive cone pigments in the mouse retina: a developmental study. J. Comp. Neurol. 331, 564–577 (1993).
McCaffery, P., Wagner, E., O'Neil, J., Petkovich, M. & Drager, U.C. Dorsal and ventral retinal territories defined by retinoic acid synthesis, break-down and nuclear receptor expression. Mech. Dev. 82, 119–130 (1999).
Li, H. et al. A retinoic acid synthesizing enzyme in ventral retina and telencephalon of the embryonic mouse. Mech. Dev. 95, 283–289 (2000).
el Akawi, Z. & Napoli, J.L. Rat liver cytosolic retinal dehydrogenase: comparison of 13-cis-, 9-cis-, and all-trans-retinal as substrates and effects of cellular retinoid-binding proteins and retinoic acid on activity. Biochemistry 33, 1938–1943 (1994).
Lin, M., Zhang, M., Abraham, M., Smith, S.M. & Napoli, J.L. Mouse retinal dehydrogenase 4 (RALDH4), molecular cloning, cellular expression and activity in 9-cis-retinoic acid biosynthesis in intact cells. J. Biol. Chem. 278, 9856–9861 (2003).
Lamb, T.D. Evolution of vertebrate retinal photoreception. Phil. Trans. R. Soc. Lond. B 364, 2911–2924 (2009).
Shichida, Y. & Matsuyama, T. Evolution of opsins and phototransduction. Phil. Trans. R. Soc. Lond. B 364, 2881–2895 (2009).
Terakita, A. The opsins. Genome Biol. 6, 213 (2005).
Bowmaker, J.K. Evolution of vertebrate visual pigments. Vision Res. 48, 2022–2041 (2008).
Lamb, T.D., Collin, S.P. & Pugh, E.N., Jr. Evolution of the vertebrate eye: opsins, photoreceptors, retina and eye cup. Nat. Rev. Neurosci. 8, 960–976 (2007).
Alvarez-Delfin, K. et al. Tbx2b is required for ultraviolet photoreceptor cell specification during zebrafish retinal development. Proc. Natl. Acad. Sci. USA 106, 2023–2028 (2009).
Pearson, B.J. & Doe, C.Q. Specification of temporal identity in the developing nervous system. Annu. Rev. Cell Dev. Biol. 20, 619–647 (2004).
Matsuda, T. & Cepko, C.L. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc. Natl. Acad. Sci. USA 101, 16–22 (2004).
Blackshaw, S. et al. Genomic analysis of mouse retinal development. PLoS Biol. 2, e247 (2004).
Barroso-Chinea, P. et al. Detection of two different mRNAs in a single section by dual in situ hybridization: a comparison between colorimetric and fluorescent detection. J. Neurosci. Methods 162, 119–128 (2007).
Peng, G.H. & Chen, S. Chromatin immunoprecipitation identifies photoreceptor transcription factor targets in mouse models of retinal degeneration: new findings and challenges. Vis. Neurosci. 22, 575–586 (2005).
Geisberg, J.V. & Struhl, K. Quantitative sequential chromatin immunoprecipitation, a method for analyzing co-occupancy of proteins at genomic regions in vivo. Nucleic Acids Res. 32, e151 (2004).
Acknowledgements
We thank D. Forrest for providing antibodies to Trβ2 and for supplying Trβ2−/− and Trβ1−/−; Trβ2−/− mice. We also thank J. Nathans, T. Shimogori, W. Yap and members of the Blackshaw laboratory for their comments on the manuscript. This work was supported by grants from the US National Institutes of Health (R01EY017015 to S.B. and RO1EY012543 to S.C.). S.B. is a W.M. Keck Distinguished Young Investigator in Medical Science.
Author information
Authors and Affiliations
Contributions
A.O., S.C. and S.B. designed the study. A.O. and G.-H.P. performed the experiments. A.O., G.-H.P. and S.C. contributed reagents and analytic tools. A.O., G.-H.P., S.C. and S.B. analyzed the data. A.O. and S.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 1 and 2 (PDF 4905 kb)
Rights and permissions
About this article
Cite this article
Onishi, A., Peng, GH., Chen, S. et al. Pias3-dependent SUMOylation controls mammalian cone photoreceptor differentiation. Nat Neurosci 13, 1059–1065 (2010). https://doi.org/10.1038/nn.2618
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2618
This article is cited by
-
Intrinsically different retinal progenitor cells produce specific types of progeny
Nature Reviews Neuroscience (2014)
-
Intrinsic control of mammalian retinogenesis
Cellular and Molecular Life Sciences (2013)
-
Plasticity of photoreceptor-generating retinal progenitors revealed by prolonged retinoic acid exposure
BMC Developmental Biology (2011)
-
Emerging roles of the SUMO pathway in development
Cellular and Molecular Life Sciences (2011)