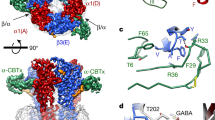
Abstract
GABAB receptors are the G-protein-coupled receptors for γ-aminobutyric acid (GABA), the main inhibitory neurotransmitter in the brain. They are expressed in almost all neurons of the brain, where they regulate synaptic transmission and signal propagation by controlling the activity of voltage-gated calcium (Cav) and inward-rectifier potassium (Kir) channels1. Molecular cloning revealed that functional GABAB receptors are formed by the heteromeric assembly of GABAB1 with GABAB2 subunits2,3,4,5. However, cloned GABAB(1,2) receptors failed to reproduce the functional diversity observed with native GABAB receptors6,7,8. Here we show by functional proteomics that GABAB receptors in the brain are high-molecular-mass complexes of GABAB1, GABAB2 and members of a subfamily of the KCTD (potassium channel tetramerization domain-containing) proteins. KCTD proteins 8, 12, 12b and 16 show distinct expression profiles in the brain and associate tightly with the carboxy terminus of GABAB2 as tetramers. This co-assembly changes the properties of the GABAB(1,2) core receptor: the KCTD proteins increase agonist potency and markedly alter the G-protein signalling of the receptors by accelerating onset and promoting desensitization in a KCTD-subtype-specific manner. Taken together, our results establish the KCTD proteins as auxiliary subunits of GABAB receptors that determine the pharmacology and kinetics of the receptor response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bettler, B., Kaupmann, K., Mosbacher, J. & Gassmann, M. Molecular structure and physiological functions of GABAB receptors. Physiol. Rev. 84, 835–867 (2004)
Jones, K. A. et al. GABAB receptors function as a heteromeric assembly of the subunits GABABR1 and GABABR2. Nature 396, 674–679 (1998)
Kaupmann, K. et al. Expression cloning of GABAB receptors uncovers similarity to metabotropic glutamate receptors. Nature 386, 239–246 (1997)
Kaupmann, K. et al. GABAB-receptor subtypes assemble into functional heteromeric complexes. Nature 396, 683–687 (1998)
White, J. H. et al. Heterodimerization is required for the formation of a functional GABAB receptor. Nature 396, 679–682 (1998)
Bonanno, G. & Raiteri, M. Multiple GABAB receptors. Trends Pharmacol. Sci. 14, 259–261 (1993)
Cruz, H. G. et al. Bi-directional effects of GABAB receptor agonists on the mesolimbic dopamine system. Nature Neurosci. 7, 153–159 (2004)
Deisz, R. A., Billard, J. M. & Zieglgänsberger, W. Presynaptic and postsynaptic GABAB receptors of neocortical neurons of the rat in vitro: differences in pharmacology and ionic mechanisms. Synapse 25, 62–72 (1997)
Berkefeld, H. et al. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science 314, 615–620 (2006)
Schwenk, J. et al. Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science 323, 1313–1319 (2009)
Zolles, G. et al. Association with the auxiliary subunit PEX5R/Trip8b controls responsiveness of HCN channels to cAMP and adrenergic stimulation. Neuron 62, 814–825 (2009)
Schuler, V. et al. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABAB responses in mice lacking GABAB1 . Neuron 31, 47–58 (2001)
Vigot, R. et al. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron 50, 589–601 (2006)
Gassmann, M. et al. Redistribution of GABAB1 protein and atypical GABAB responses in GABAB2-deficient mice. J. Neurosci. 24, 6086–6097 (2004)
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nature Biotechnol. 26, 1367–1372 (2008)
Maurel, D. et al. Cell-surface protein–protein interaction analysis with time-resolved FRET and snap-tag technologies: application to GPCR oligomerization. Nature Methods 5, 561–567 (2008)
Kulik, A. et al. Compartment-dependent colocalization of Kir3.2-containing K+ channels and GABAB receptors in hippocampal pyramidal cells. J. Neurosci. 26, 4289–4297 (2006)
Sodickson, D. L. & Bean, B. P. GABAB receptor-activated inwardly rectifying potassium current in dissociated hippocampal CA3 neurons. J. Neurosci. 16, 6374–6385 (1996)
Arikkath, J. & Campbell, K. P. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr. Opin. Neurobiol. 13, 298–307 (2003)
Jentsch, T. J. CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit. Rev. Biochem. Mol. Biol. 43, 3–36 (2008)
Rettig, J. et al. Inactivation properties of voltage-gated K+ channels altered by presence of β-subunit. Nature 369, 289–294 (1994)
Duthey, B. et al. A single subunit (GB2) is required for G-protein activation by the heterodimeric GABAB receptor. J. Biol. Chem. 277, 3236–3241 (2002)
Nicoll, R. A. My close encounter with GABAB receptors. Biochem. Pharmacol. 68, 1667–1674 (2004)
Otis, T. S., De Koninck, Y. & Mody, I. Characterization of synaptically elicited GABAB responses using patch-clamp recordings in rat hippocampal slices. J. Physiol. (Lond.) 463, 391–407 (1993)
Price, C. J., Scott, R., Rusakov, D. A. & Capogna, M. GABAB receptor modulation of feedforward inhibition through hippocampal neurogliaform cells. J. Neurosci. 28, 6974–6982 (2008)
Tamas, G., Lorincz, A., Simon, A. & Szabadics, J. Identified sources and targets of slow inhibition in the neocortex. Science 299, 1902–1905 (2003)
Wischmeyer, E. et al. Subunit interactions in the assembly of neuronal Kir3.0 inwardly rectifying K+ channels. Mol. Cell. Neurosci. 9, 194–206 (1997)
Schaeren-Wiemers, N. & Gerfin-Moser, A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry 100, 431–440 (1993)
Rubinson, D. A. et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nature Genet. 33, 401–406 (2003)
Engle, M. P. et al. Spinal nerve ligation does not alter the expression or function of GABAB receptors in spinal cord and dorsal root ganglia of the rat. Neuroscience 138, 1277–1287 (2006)
Rivas, G., Stafford, W. & Minton, A. P. Characterization of heterologous protein–protein interactions using analytical ultracentrifugation. Methods 19, 194–212 (1999)
Kulik, A. et al. Subcellular localization of metabotropic GABAB receptor subunits GABAB1a/b and GABAB2 in the rat hippocampus. J. Neurosci. 23, 11026–11035 (2003)
Masugi-Tokita, M. et al. Number and density of AMPA receptors in individual synapses in the rat cerebellum as revealed by SDS-digested freeze-fracture replica labeling. J. Neurosci. 27, 2135–2144 (2007)
Zolles, G. et al. Pacemaking by HCN channels requires interaction with phosphoinositides. Neuron 52, 1027–1036 (2006)
Dittert, I. et al. Improved superfusion technique for rapid cooling or heating of cultured cells under patch-clamp conditions. J. Neurosci. Methods 151, 178–185 (2006)
Acknowledgements
Author Contributions
We thank J. P. Adelman and H. R. Brenner for discussions and critical reading of the manuscript, and A. Haupt and K. Kaupmann for help with bioinformatics and shRNA experiments, respectively. This work was supported by grants from the Deutsche Forschungsgemeinschaft to B.F. (SFB 746/TP16; SFB 780/A3; EXC294) and to A.K. (SFB 780/A2), by grants from the Wellcome Trust (ISRF), the EU Synapse and the GACR (309/06/1304) to R.T., and by grants from the Swiss Science Foundation (3100A0-117816), the Fridericus Stiftung and the European Community’s 7th Framework Programme (FP7/2007-2013) under Grant Agreement 201714 to B.B.
Author information
Authors and Affiliations
Contributions
J.S., M.M., G.Z. and R.T. are equally contributing first authors. J.S., W.B., V.R., U.S. and B.F. performed proteomic analysis, biochemistry and evaluation of mass spectrometry. M.M., J.Y.T., M.G., J.T., T.F., M.R. and K.I. performed in situ hybridization, cellular biology, mouse work and KCTD antibody generation. G.Z., R.T., R.S. and M.R. conducted the electrophysiological recordings on oocytes, cultured cells and neurons. A.U., E.T. and A.K. performed electron microscopy. B.F., B.B. and U.S. initiated, designed and supervised the study. B.F. and B.B. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-13 with legends and Supplementary Tables 1-2. (PDF 5718 kb)
Rights and permissions
About this article
Cite this article
Schwenk, J., Metz, M., Zolles, G. et al. Native GABAB receptors are heteromultimers with a family of auxiliary subunits. Nature 465, 231–235 (2010). https://doi.org/10.1038/nature08964
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08964
This article is cited by
-
Profiling the proximal proteome of the activated μ-opioid receptor
Nature Chemical Biology (2024)
-
Specific pharmacological and Gi/o protein responses of some native GPCRs in neurons
Nature Communications (2024)
-
Calpain activity is negatively regulated by a KCTD7–Cullin-3 complex via non-degradative ubiquitination
Cell Discovery (2023)
-
A slit-diaphragm-associated protein network for dynamic control of renal filtration
Nature Communications (2022)
-
TTYH family members form tetrameric complexes at the cell membrane
Communications Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.