Abstract
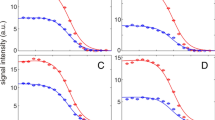
The characterisation of non-native states of proteins is a key problem instudies of protein folding. Complete characterisation of these states requiresa description of both local and global properties, including moleculardimensions. Here we present results from pulsed field gradient experimentsdesigned to compare the effective hydrodynamic radii of a protein in nativeand non-native states. Measurements performed on lysozyme indicate that theeffective hydrodynamic radius increases by 38±1% on unfolding in urea,a result completely consistent with a recent study by small-angle X-rayscattering.
Similar content being viewed by others
References
Altieri, A.S., Hinton, D.P. and Byrd, R.A. (1995) J. Am. Chem. Soc., 117, 7566–7567.
Arêas, E.P.G., Mauro, C.C. and Santos, P.S. (1995) J. Mol. Struct., 378, 111–119.
Balbach, J., Forge, V., Van Nuland, N.A.J., Winder, S., Hore, P.J. and Dobson, C.M. (1995) Nat. Struct. Biol., 2, 865–870.
Buschmann, J., Muller, E. and Luger, P. (1986) Acta Crystallogr., C42, 873–876.
Chen, A.D., Wu, D.H. and Johnson Jr., C.S. (1995a) J. Phys. Chem., 99, 828–834.
Chen, A., Wu, D. and Johnson Jr., C.S. (1995b) J. Am. Chem. Soc., 117, 7965–7970.
Chen, L., Hodgson, K.O. and Doniach, S. (1996) J. Mol. Biol., 261, 658–671.
Dobson, C.M. and Ptitsyn, O.B. (1997) Curr. Opin. Struct. Biol., 7, 1–2.
Gast, K., Nüppert, A., Müller-Frohne, M., Zirwer, D. and Damaschun, G. (1997) Eur. Biophys. J., 25, 211–219.
Gibbs, S.J. and Johnson Jr., C.S. (1991) J. Magn. Reson., 93, 395–402.
Jones, J.A., Hodgkinson, P.J., Barker, A.L. and Hore, P.J. (1996) J. Magn. Reson., B113, 25–34.
Lattman, E.E. (1994) Curr. Opin. Struct. Biol., 4, 87–92.
Lin, M. and Larive, C.K. (1995) Anal. Biochem., 229, 214–220.
Morris, K.F. and Johnson Jr., C.S. (1992) J. Am. Chem. Soc., 114, 3139–3141.
Pace, C.N. (1986) Methods Enzymol., 131, 266–280.
Plaxco, K.W., Morton, C.J., Grimshaw, S.B., Jones, J.A., Pitkeathly, M., Campbell, I.D. and Dobson, C.M. (1997) J. Biomol. NMR, in press.
Shortle, D.R. (1996) Curr. Opin. Struct. Biol., 6, 24–30.
Sklenář, V., Torchia, D. and Bax, A. (1987) J. Magn. Reson., 73, 375–379.
Smith, L.J., Fiebig, K.M., Schwalbe, H. and Dobson, C.M. (1996) Folding Design, 1, R95–R106.
Steiner, R.F. (1964) Biochim. Biophys. Acta, 79, 51–63.
Stejskal, E.O. and Tanner, J.E. (1965) J. Chem. Phys., 42, 288–292.
Van den Bos, A. (1982) In Handbook of Measurement Science, Vol. 2 (Ed., Sydenham, P.H.), Wiley, New York, NY, U.S.A., pp. 331–377.
Wu, D., Chen, A. and Johnson Jr., C.S. (1996) J. Magn. Reson., A123, 215–218.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jones, J.A., Wilkins, D.K., Smith, L.J. et al. Characterisation of protein unfolding by NMR diffusion measurements. J Biomol NMR 10, 199–203 (1997). https://doi.org/10.1023/A:1018304117895
Issue Date:
DOI: https://doi.org/10.1023/A:1018304117895