Abstract
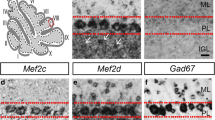
The recent identification of decreased protein levels of glutamate decarboxylase (GAD) 65 and 67 isoforms in the autistic cerebellar tissue raises the possibility that abnormal regulation of GABA production in individual neurons may contribute to the clinical features of autism. Reductions in Purkinje cell number have been widely reported in autism. It is not known whether the GAD changes also occur in Purkinje cells at the level of transcription. Using a novel approach, the present study quantified GAD67 mRNA, the most abundant isoform in Purkinje cells, using in situ hybridization in adult autistic and control cases. The results indicate that GAD67 mRNA level was reduced by 40% in the autistic group (P < 0.0001; two-tailed t test), suggesting that reduced Purkinje cell GABA input to the cerebellar nuclei potentially disrupts cerebellar output to higher association cortices affecting motor and/or cognitive function. These findings may also contribute to the understanding of previous reports of alterations in the GABAergic system in limbic and cerebro-cortical areas contributing to a more widespread pathophysiology in autistic brains.



Similar content being viewed by others
References
Aggensteiner M, Reiser G (2003) Expression of the brain-specific membrane adapter protein p42IP4/centaurin alpha, a Ins(1,3,4,5)P4/PtdIns(3,4,5)P3 binding protein, in developing rat brain. Dev Brain Res 142:77–87
Arin DM, Bauman ML, Kemper TL (1991) The distribution of Purkinje cell loss in the cerebellum in autism. Neurology 41(Suppl):307
Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K (1997) Cleft palate and decrased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci USA 94(12): 6496–6499
Bauman ML, Kemper TL (1985) Histoanatomic observations of the brain in early infantile autism. Neurology 35:866–874
Bauman ML, Kemper TL (1994) Neuroanatomic observations of the brain in autism. In: Bauman ML, Kemper TL (eds) The neurobiology of autism. John Hopkins University Press, Baltimore, pp 119–145
Bauman ML, Kemper TL (2005) Neuroanatomic observations of the brain in autism: a review and future directions. Int J Dev Neurosci 23:183–187
Blatt GJ, Fitzgerald CM, Guptill JT, Booker AB, Kemper TL, Bauman ML (2001) Density and distribution of hippocampal neurotransmitter receptors in autism: an autoradiographic study. J Autism Dev Disord 31(6):537–543
Blatt GJ (2005) GABAergic cerebellar system in autism: a neuropathological and developmental perspective. Int Rev Neurobiol 71:167–178
Brooksbank BW, Atkinson DJ, Balazs R (1981) Biochemical development of the human brain. II. Some parameters of the GABAergic system. Dev Neurosci 4(3):188–200
Bu DF, Erlander MG, Hiltz BC, Tillakaratne NJK, Kaufman DI, Wagber-McPherson CB, Evan GA, Tobin AJ (1992) Two human glutamate decarboxylase, 65 kDa GAD and 67 kDa GAD, are each encoded by a single gene. Proc Natl Acad Sci USA 89:2115–2119
Buxbaum JD, Silverman JM, Smith CJ, Kilifarski M, Reichert J, Hollander E, Lawlor BA, Fitzgerald M, Greenberg DA, Davis KL (2001) Evidence for a susceptibility gene for autism chromosome 2 for genetic heterogeneity. Am J Hum Genet 68(6):1514–1520
Center for Disease Control (CDC): 2006 autismspeaks.org
Chan-Palay V, Palay SL, Wu JY (1979) Gamma-aminobutyric acid pathways in the cerebellum studied by retrograde and anterograde transport of glutamic acid decarboxylase antibody after in vivo injections. Anat Embryol (Berl) 157(1):1–14
Chesselet MF, Weiss L, Wuenschell C, Tobin AJ, Affolter H-U (1987) Comparative distribution of mRNAs for glutamic acid decarboxylase, tyrosine hydroxylase, and tachykinins in the basal ganglia: an in situ hybridization study in the rodent brain. J Comp Neurol 262:125–140
Cox KH, DeLeon DV, Angerer LM, Angerer RC (1984) Detection of mRNAs in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol 101:485–502
Crepel F, Mariani J (1976) Multiple innervation of Purkinje cells by climbing fibers in the cerebellum of the weaver mutant mouse. J Neurobiol 7(6):579–582
Crepel F, Delhaye-Bouchaud N, Guastavino JM, Sampaio I (1980) Multiple innervation of cerebellar Purkinje cells by climbing fibers in staggerer mutant mouse. Nature 283(5746):483–484
Dhossche D, Applegate H, Abraham A, Maertens P, Bland L, Bencsath A, Martinez J (2002) Elevated plasma GABA levels in autistic youngsters: stimulus for a GABA hypothesis in autism. Med Sci Monitor 8(8):PR1–PR6
Drengler SM, Oltman GA (1993) Rapid increases in cerebellar Purkinje cell glutamic acid decarboxylase GAD67 mRNA after lesion-induced increases in cell firing. Brain Res 615(1):175–179
Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ (1991a) Two genes encode distinct glutamate decarboxylases. Neuron 7:91–100
Erlander MG, Tobin AJ (1991b) The structural and functional heterogeneity of glutamate decarboxylase. A review. Neurochem Res 16:215–226
Esclapez M, Tillakaratne NJK, Tobin AJ, Houser CR (1993) Comparative localization of mRNAs encoding two forms of glutamic acid decarboxylase with non radioactive in situ hybridization methods. J Comp Neurol 331:339–362
Esclapez M, Tillakaratne NJ, Kaufman DL, Tobin AJ, Houser CR (1994) Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms. J Neurosci 14(3 Pt 2):1834–1855
Fatemi SH, Halt AR, Realmuto G, Earle J, Kist DA, Thuras P, Mer A (2002a) Purkinje cell size is reduced in cerebellum of patients with autism. Cell Mol Neurobiol 22(2):171–175
Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR (2002b) Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psych 52:805–810
Feldblum S, Erlander MG, Tobin AJ (1993) Different distributions of GAD65 and GAD67 mRNAs suggest that the two glutamate decarboxylases play distinctive functional roles. J Neurosci Res 34(6):689–706
Greger V, Knoll JH, Woolf F, Glatt K, Tyndale RF, DeLorey TM, Olsen RW, Tobin AJ, Sikela JM, Nakatsu Y et al (1995) The gamma-aminobutyric acid receptor gamma 3 subunit gene (GABARG3) is tightly linked to the alpha 5 subunit gene (GABRA5) on human chromosome 15q11-q13 and is transcribed in the same orientation. Genomics 26(2):258–264
Gepner B, Mestre DR (2002) Postural reactivity to fast visual motion differentiates autistic from children with Asperger syndrome. J Autism Dev Disord 32:231–238
Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevinin V, Dwivedi Y, Grayson DR, Impagnateillo F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E (2000) Decrease in reelin and glutamic acid decarboxylase 67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry 57(11):1061–1069
Hamilton SP, Woo JM, Carlson EJ, Ghanem E, Ekker M, Rubenstein JLR (2005) Analysis of four DLX homeobox genes in autistic probands. BMC Genetics 6:52
Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, Sun Z, Sampson AR, Lewis DA (2005) Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci 25(2):372–383
Heckers S, stone D, Walsh J, Shick J, Koul P, Benes FM (2002) Differential hippocampal expression glutamic acid decarboxylase 65 and 67 messenger RNA in bipolar disorder and schizophrenia. Arch Gen Psych 59(6):521–529
Hynd M, Lewohl JM, Scott HL, Dodd PR (2003) Biochemical and molecular studies using human autopsy brain tissue. J Neurochem 85:543–562
IMGSAC (2001) A genomewide screen for autism: strong evidence for linkage to chromosome 2q, 7q, and 16p. Am J Hum Genet 69:570–581
Impagnatiello F, Guidotti AR, Pesold C, Dwivedi Y, Caruncho H, Pisu MG, Uzunov DP, Smalheiser NR, Davis JM, Pandey GN, Pappas GD, Tueting P, Sharma RP, Costa E (1998) A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci USA 95(26):15718–15723
Jeneskog T, Padel Y (1984) An excitatory pathway through dorsal columns to rubrospinal cells in the cat. J Physiol 353:355–373
Jones RS (1988) Epileptiform events induced by GABA-antagonists in entorhinal cortical cells in vitro are partly mediated by NMDA receptors. Brain Res 457(1):113–121
Jones MW, Kilpatrick IC, Phillipson OT (1988) Dopamine function in the prefrontal cortex of the rat is sensitive to a reduction of tonic GABA-mediated inhibition in the thalamic mediodorsal nucleus. Exp Brain Res 69(3):623–634
Kalkman HO, Loetscher (2003) GAD67: the link between the GABA-deficit hypothesis and the dopaminergic- and glutamatergic theories of psychosis. J Neural Transm 110:803–812
Kanner L (1943) Autistic disturbances of affective contact. Nerv Child 2:217–250
Katz J, Nielsen KM, Soghomonian JJ (2005) Comparative effects of acute or chronic administration of levodopa to 6-hydroxydopamine-lesioned rats on the expression of glutamic acid decarboxylase in the neostriatum and GABAA receptors subunits in the substantia nigra pars reticulata. Neurosci 132(3):833–842
Kaufman DL, Houser CR, Tobin AJ (1991) Two forms of the gamma-aminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J Neurochem 56(2):720–723
Kemper TL, Bauman ML (1998) Neuropathology of infantile autism. J Neuropathol Exp Neurol 57(7):645–652
Kern JK (2003) Purkinje cell vulnerability and autism: a possible etiological connection. Brain Dev 25(6):377–382
Laprade N, Soghomonian JJ (1995) Differential regulation of mRNA levels encoding for the two isoforms of glutamate decarboxylase (GAD65 and GAD67) by dopamine receptors in the rat striatum. Brain Res Mol Brain Res 34(1):65–74
Lauder JM, Han VKM, Henderson P, Verdoorn T, Towle AC (1986) Prenatal ontogeny of the GABAergic system in the rat brain: an immunocytochemical study. Neurosci 2:4658–4693
Lauritsen M, Mors O, Mortensen PB, Ewald H (1999) Infantile autism and associated autosomal chromosome abnormalities: a register-based study and a literature survey. J Child Psychol Psych 40(3):335–345
Legay F, Pelhate S, Tappaz M.L. (1982) Phylogenesis of brain glutamic acid decarboxylase from vertebrates: Immunohistochemical studies. J Neurochem 46:1478–1486
Levisohn L, Cronin-golomb A, Schmahmann JD (2000) Neuropsychological consequence of cerebellar tumor resection in children: cerebellar cognitive affective syndrome in a pediatric population. Brain 123:1041–1050
Lipska BK, Weinberger DR (2000) To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacol 23(3):223–239
Llinas RR, Walton KD, Lang EJ (2003) Cerebellum. In: Shepherd GM (eds) The synaptic organization of the brain, 5th ed. Oxford University Press, New York, pp 271–309
Ma DQ, Whitehead PL, Menold MM, Martin ER, Ashley-Koch AE, Mei H, Ritchie MD, Delong GR, Abramson RK, Wright HH, Cuccaro ML, Hussman JP, Gilbert JR, Pericak-Vance MA (2005) Identification of significant association and gene–gene interaction of GABA receptor subunit genes in autism. Am J Hum Genet 77:377–388
Maddox LO, Menold MM, Bass MP, Rogala AR, Pericak-Vance MA, Vance JM, Gilbert JR (1999) Autistic disorder and chromosome 15q11-q13: construction and analysis of a BAC/PAC contig. Genomics 62(3):325–331
Maniatis T, Frisch EF, Sambrook P (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, New York
Martin ER, Menold MM, Wolpert CM, Bass MP, Donnelly SL, Ravan SA, Zimmerman A, Gilbert JR, Vance JM, Maddox LO, Wright HH, Abramson RK, Delong GR, Cuccaro ML, Pericak-Vance MA (2000) Analysis of linkage disequilibrium in GABA receptor subunit genes in autistic disorder. Am J Med Genet 96:43–48
Mari M, Castiello U, Marks D, Marraffa C, Prior M (2003) The reach to grasp movement in children with autism spectrum disorder. Phil Trans R. Soc Lond B 358:393–403
Mariani J (1982) Extent of multiple innervation of Purkinje cells by climbing fibers in the olivocerebellar system of weaver, reeler, and staggerer mutant mice. J Neurobiol 13(2):119–126
Mengod G, Goudsmit E, Probst A, Palacios JM (1992) In situ hybridization in the human hypothalamus. Prog Brain Res 93:45–55
Moffett JR, Palkovits M, Namboodiri A, Neale JH (1994) Comparative distribution of N-acetlyaspartylglutamate and GAD67 in the cerebellum and precerebellar nuclei of the rat utilizing enhanced carboiimide fixation and immunohistochemistry. J Comp Neurol 347(4):598–618
Muhle R, Trentacoste SV, Rapin I (2004) The genetics of autism. Pediatrics 113(5):472–486
Olney JW, Farber NB (1995) NMDA antagonists as neurotherapeutic drugs, psychotogens, neurotoxins, and research tools for studying schizophrenia. Neuropsychopharmacol 13(4):335–345
Palacios JM, Mengod G (1992) Visualization of neurotransmitter receptors and their mRNAs in the human brain. Arzneimittelforschung 42(2A):189–195
Palay SL, Chan-Palay V (1974) Cerebellar cortex: cytology and organization. Springer, Berlin Heidelberg New York
Philippe A, Martinez M, Bataille-Guillot M, Gillberg C, Rastam M, Sponheim E, Coleman M, Zappella M, Aschauer H, Van Maldergerm LV, Penet C, Feingold J, Brice A, Leboyer M, the Paris Autism Research International Sibpair study. (1999) An autosomal genomic screen for autism. Collaborative linkage study of autism. Am J Hum Genet 68:1514–1520
Pierce K, Courchesne E (2001) Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol Psych 49:655–664
Preece P, Virley DJ, Costandi M, Coombes R, Moss SJ, Mudge AW, Jazin E, Cairns NJ (2003) An optimistic view for quantifying mRNA in post mortem human brain. Mol Brain Res 116:7–16
Rabionet R, Jaworski JM, Ashley-Koch AE, Martin ER, Sutcliffe JS, Haines JL, Delong GR, Abramson RK, Wright HH, Cuccaro ML, Gilbert JR, Pericak-Vance MA (2004) Analysis of the autism chromosome 2 linkage region: GAD1 and other candidate genes. Neurosci Lett 372:209–214
Ramnani N (2006) The primate cortico-cerebellar system—anatomy and function. Nat Neurosci Rev 7(7):511–522
Rapin I (1991) Autistic children: diagnosis and clinical feature. Pediatrics 87:751–760
Rapin I, Katzman R (1998) Neurobiology of autism. Ann Neurol 43(1):7–14
Rimvall K, Sheikh SN, Martin DL (1993) Effects of increased γ-aminobutyric acid levels on GAD67 protein and mRNA levels in rat cerebral cortex. J Neurochem 60:714–720
Rimvall K, Martin DL (1994) The level of GAD67 protein is highly sensitive to small increases in intraneuronal γ-aminobutyric acid levels. J Neurochem 62:1375–1381
Rinehart NJ, Bradshaw JL, Brereton AV, Tonge BJ (2001) Movement preparation in high functioning autism and Asperger disorder: a serial choice reaction time task involving motor reprogramming. J Autism Dev Disord 31(1):79–88
Risch N, Spiker D, Lotspeich L, Nouri N, Hinds D, Hallmayer J, Kalaydjieva L, McCague P, Dimiceli S, Pitts, Nguyen L, Yang J, Harper C, Thorpe D, Vermeer S, Young H, Herbert J, Lin A, Ferguson J, Chiotti C, Wiese-Slater S, Rogers T, Salmon B, Nicholas P, Petersen PB, Pingree C, McMahon W, Wong DL, Cavalli-Sforza LL, Kraemer HC, Myers RM (1999) A genomic screen of autism: evidence for a multilocus etiology. Am J Hum Genet 65(2):493–507
Riva D, Giorgi (2000) The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumors. Brain 123:1051–1061
Schmahmann JD, Doyon J, Toga AW, Petrides M, Evans AC (2000) MRI atlas of the cerebellum. Academic, New York
Schmahmann JD, Kiliany RJ, Moore TL, DeMong C, MacMore JP, Moss MB (2004) Cerebellar dentate nucleus lesions impair flexibility but not motor function in monkeys. Soc Neurosci Abstr 34:254–312
Schroer RJ, Phelan MC, Michaelis RC, Crawford EC, Skinner SA, Cuccaro M, Simensen RJ, Bishop J, Skinner C, Fender D, Stevensone RE (1998) Autism and maternally derived aberrations of chromosome 15q. Am J Med Genet 76:327–336
Segovia J, Tillakaratne NJ, Whelan K, Tobin AJ, Gale K (1990) Parallel increases in striatal glutamic acid decarboxylase activity and mRNA levels in rats with lesions of the nigrostriatal pathway. Brain Res 529:345–348
Shao Y, Wolpert CM, Raiford KL, Menold MM, Donnelly SL, Ravan SA, Bass MP, McClain C, Von Wendt L, Vance JM, Abramson RH, Wright HH, Ashley-Koch A, Gilbert JR, DeLong RG, Cuccaro ML, Pericak-Vance MA (2002) Genomic screen and follow-up analysis for autistic disorder. Am J Med Genet 114:99–105
Stuhmer T, Anderson SA, Ekker M, Rubenstein KL (2002) Ectopic expression of the DLX genes induces glutamic acid decarboxylase and DLX expression. Development 129:245–252
Soghomonian JJ, Gonzales C, Chesselet MF (1992) Messenger RNAs encoding glutamate-decarboxylases are differentially affected by nigrostriatal lesions in subpopulations of striatal neurons. Brain Res 576(1):68–79
Soghomonian JJ (1993) Effects of neonatal 6-hydroxydopamine injections on glutamate decarboxylase, preproenkephalin and dopamine D2 receptor mRNAs in the adult rat striatum. Brain Res 621:249–259
Soghomonian JJ, Pedneault S, Audet G, Parent A (1994) Increased glutamate decarboxylase mRNA levels in the striatum and pallidum of MPTP-treated primates. J Neurosci 14:6256–6265
Soghomonian JJ, Laprade N (1997) Glutamate decarboxylase (GAD67 and GAD65) gene expression is increased in a subpopulation of neurons in the putamen of Parkinsonian monkeys. Synapse 27:122–132
Soghomonian JJ, Martin DL (1998) Two isoforms of glutamate decarboxylase: why? Trends Pharmacol Sci 19(12):500–505
Teune TM, Van der Burg J, Van der Moer J, Voodg J, Ruigrok TJ (2000) Topography of cerebellar nuclear projections to the brain stem in the rat. Prog Brain Res 124:141–172
Torrey FF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB (2005) Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psych 57(3):252–260
Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA (2000) Decreased glutamic acid decoarbosylase 67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry 57(3):237–245
Walker E, McNicol AM (1992) In situ hybridization demonstrates the stability of mRNA in post mortem rat tissues. J Pathol 168(1):67–73
Welsh JP, Yuen G, Placantonakis DG, Vu TQ, Haiss F, O’Hearn E, Molliver ME, Aicher SA (2002) Why do Purkinje cells die so easily after global brain ischemia? Aldolase C, EAAT4 and the cerebellar contribution to post-hypoxic myoclnus. Adv Neurol 89:331–359
Whitney ER, Kemper TL, Bauman ML, Blatt GJ (2004) Calcium binding proteins in cerebellar Purkinje cells in autistic cerebellum. Soc Neurosci Abstr 34:116.11
Willcutts MD, Griffin WST, Morrison-Bogorad M (1989) Analysis of glutamic acid decarboxylase mRNA levels during cerebellar development in rat. Neurosci Res Commun 6:57–65
Willcutts MD, Morrison-Bogorad M (1991) Quantitative in situ hybridization analysis of glutamic acid decarboxylase messenger RNA in developing rat cerebellum. Dev Brain Res 63:253–164
Woo TU, Walsh JP, Benes FM (2004) Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-d-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry 61(7):649–657
Yip J, Marcon R, Kemper TL, Bauman ML, Blatt GJ. (2005): The olivocerebellar projection in autism: using the intermediate filament protein peripherin as a marker for climbing fibers. International Meeting for Autism Res Abstr (IMFAR) 5:49
Yip J, Soghomonian JJ, Bauman M, Kemper T, Blatt GJ (2006) Functional status of the cerebellar Purkinje cells in autistic brains. International Meeting for Autism Research (IMFAR) 5:142
Acknowledgments
We gratefully acknowledge the Harvard Brain Tissue Resource Center, the Autism Tissue Program (ATP) and the University of Miami and Maryland Brain Banks for providing brain tissues for this study. This work is supported by grants NIH NICHD #HD39459-04 and the Hussman Foundation (GJB, P.I.). We thank Linh Nguyen for her excellent technical assistance with in situ hybridization and Rita Marcon for assistance with tissue cutting.
Author information
Authors and Affiliations
Corresponding author
Additional information
All the authors contributed equally.
Rights and permissions
About this article
Cite this article
Yip, J., Soghomonian, JJ. & Blatt, G.J. Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: pathophysiological implications. Acta Neuropathol 113, 559–568 (2007). https://doi.org/10.1007/s00401-006-0176-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-006-0176-3