Abstract
Rationale
3α-OH-5α[β]-pregnan-20-one (THP) is a positive modulator of the GABAA receptor (GABAR), which underlies its reported anxiolytic effect. However, there are conditions such as premenstrual dysphoric disorder (PMDD) where increases in THP levels can be associated with adverse mood.
Objectives
In order to test for conditions where THP might be anxiogenic, we developed a mouse model of THP withdrawal. Because δ-containing GABAR are highly sensitive to THP modulation, results were compared in wild-type and δ knockout mice.
Methods
Finasteride, a 5α-reductase blocker, was administered for 3 days to female wild-type or δ knockout mice. Then, animals were tested in the elevated plus maze, following acute administration of THP, lorazepam, flumazenil, or 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP), and results compared to vehicle-injected controls. CA1 hippocampal GABAR α4 subunit levels were assessed by Western blot.
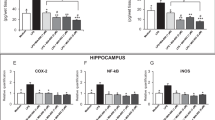
Results
After THP withdrawal, THP produced anxiogenic effects, decreasing open arm entries on the elevated plus maze, following a brief shock, in contrast to its expected anxiolytic effects. As we have shown in rats, THP withdrawal also resulted in increased expression of the α4 subunit in mouse CA1 hippocampus. As expected for increases in α4-containing GABAR, THP withdrawn mice were relatively insensitive to the benzodiazepine (BDZ) lorazepam and had atypical responses to the BDZ antagonist flumazenil when tested on the plus maze. In contrast, they showed a greater anxiolytic response to THIP, which has greater efficacy at α4βδ than other GABAR. Although THP withdrawal in δ knockout mice also increased the α4 GABAR subunit, the anxiogenic effects of THP and the anxiolytic effects of THIP were not observed, implicating α4βδ GABAR in these effects.
Conclusions
Based on these behavioral and pharmacological findings, we suggest that THP withdrawal in the mouse may serve as a rodent model of PMDD.




Similar content being viewed by others
References
Akwa Y, Purdy RH, Koob GF, Britton KT (1999) The amygdala mediates the anxiolytic-like effect of the neurosteroid allopregnanolone in rat. Behav Brain Res 106:119–125
Andreen L, Sundstrom-Poromaa I, Bixo M, Andersson A, Nyberg S, Backstrom T (2004) Relationship between allopregnanolone and negative mood in postmenopausal women taking sequential hormone replacement therapy with vaginal progesterone. Psychoneuroendocrinology 30:212–224
Angst J, Sellaro R, Merikangas KR, Endicott J (2001) The epidemiology of premenstrual psychological symptoms. Acta Psychiatr Scand 104:110–116
Backstrom T, Andreen L, Birzniece V, Bjorn I, Johannson IM, Nordenstam-Haghjo M, Nyberg S, Sundstrom-Poromaa I, Wahlstrom G, Wang M, Zhu D (2003a) The role of hormones and hormonal treatments in premenstrual syndrome. CNS Drugs 17:325–342
Backstrom T, Andersson A, Andreen L, Birzniece V, Bixo M, Bjorn I, Haage D, Isaksson M, Johansson IM, Lindblad C, Lundgren P, Nyberg S, Odmark IS, Stromberg J, Sundstrom-Poromaa I, Turkmen S, Wahlstrom G, Wang M, Wihlback AC, Zhu D, Zingmark E (2003b) Pathogenesis in menstrual cycle-linked CNS disorders. Ann N Y Acad Sci 1007:42–53
Belelli D, Casula A, Ling A, Lambert JJ (2002) The influence of subunit composition on the interaction of neurosteroids with GABA (A) receptors. Neuropharmacology 43:651–661
Bianchi MT, Macdonald RL (2003) Neurosteroids shift partial agonist activation of GABA (A) receptor channels from low- to high-efficacy gating patterns. J Neurosci 23:10934–10943
Bicikova M, Dibbelt L, Hill M, Hampl R, Starka L (1998) Allopregnanolone in women with premenstrual syndrome. Horm Metab Res 30:227–230
Bitran D, Shiekh M, McLeod M (1995) Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABA-A receptors. J Neuroendocrinol 7:171–177
Bitran D, Dugan M, Renda P, Ellis R, Foley M (1999) Anxiolytic effects of the neuroactive steroid pregnanolone (3alpha-OH-5beta-pregnan-20-one) after microinjection in the dorsal hippocampus and luteal septum. Brain Res 850:217–224
Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA (2002) Pharmacological characterization of a novel cell line expressing human alpha (4)beta (3)delta GABA (A) receptors. Br J Pharmacol 136:965–974
Buchanan CM, Eccles JS, Becker JB (1992) Are adolescents the victims of raging hormones: evidence for activational effects of hormones on moods and behavior at adolescence. Psychol Bull 111:62–107
Cameron JL (2004) Interrelationships between hormones, behavior, and affect during adolescence: understanding hormonal, physical, and brain changes occurring in association with pubertal activation of the reproductive axis. Introduction to part III. Ann N Y Acad Sci 1021:110–123
Cloitre M, Yonkers KA, Pearlstein T, Althemus M, Davidson KW, Pigott TA, Shear MK, Pine D, Ross J, Howell H, Brogan K, Rieckmann N, Clemow L (2004) Women and anxiety disorders: implications for diagnosis and treatment. CNS Spectr 9:1–16
Corpechot C, Young J, Clavel M, Wehrey C, Veltz JN, Touter G, Mouren M, Prasad VV, Banner C, Sjovall J, Baulieu E-E, Robel P (1993) Neurosteroids: 3α-OH-5α-pregnan-20-one and its precursors in the brain plasma and steroidogenic glands of male and female rats. Endocrinology 133:1003–1009
Corpechot C, Collins B, Carey M, Tsouros A, Robel P, Fry J (1997) Brain neurosteroids during the mouse oestrous cycle. Brain Res 766:276–280
Dunn SMJ, Tancowny B, Martin IL (2003) Characterisation of GABA-A receptors containing alpha4-beta1-delta subunits. J Physiol 548P:5P
Elliott H (2002) Premenstrual dysphoric disorder. A guide for the treating clinician. N C Med J 63:72–75
Endicott J, Amsterdam J, Eriksson E, Frank E, Freeman E, Hirschfeld R, Ling F, Parry B, Pearlstein T, Rosenbaum J, Rubinow D, Schmidt P, Severino S, Steiner M, Stewaart D, Thys-Jacobs S (1999) Is premenstrual dysphoric disorder a distinct clinical entity? J Womens Health Gend Based Med 8:663–679
Fancsik A, Linn DM, Tasker JG (2000) Neurosteroid modulation of GABA IPSCs is phosphorylation dependent. J Neurosci 20:3067–3075
Follesa P, Serra M, Cagetti E, Pisu MG, Porta S, Floris S, Massa F, Sanna E, Biggio G (2000) Allopregnanolone synthesis in cerebellar granules cells: roles in regulation of GABA (A) receptor expression and function during progesterone treatment and withdrawal. Mol Pharmacol 57:1262–1270
Freeman EW (2003) Premenstrual syndrome and premenstrual dysphoric disorder: definitions and diagnosis. Psychoneuroendocrinology 28:25–37
Freeman EW (2004) Luteal phase administration of agents for the treatment of premenstrual dysphoric disorder. CNS Drugs 18:453–468
Freeman EW, Frye CA, Rickels K, Martin PA, Smith SS (2002) Allopregnanolone levels and symptom improvement in severe premenstrual syndrome. J Clin Psychopharmacol 22:516–520
Frye CA (1995) The neurosteroid 3α,5α-THP has antiseizure and possible neuroprotective effects in an animal model of epilepsy. Brain Res 696:113–120
Frye CA, Bayon LE (1999) Cyclic withdrawal from endogenous and exogenous progesterone increases kainic acid and perforant pathway induced seizures. Pharmacol Biochem Behav 62:315–321
Frye CA, Walf AA (2004) Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav 41:306–315
Frye CA, Bayon LE, Pursnani NK, Purdy RH (1998) The neurosteroids, progesterone and 3alpha,5alpha-THP, enhance sexual motivation, receptivity, and proceptivity in female rats. Brain Res 808:72–83
Gallo MA, Smith SS (1993) Progesterone withdrawal decreases latency to and increases duration of electrified prod burial: a possible rat model of PMS anxiety. Pharmacol Biochem Behav 46:897–904
Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL (2001) Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry 49:788–797
Griffiths J, Lovick T (2005) Withdrawal from progesterone increases expression of alpha4, beta1 and delta GABA(A) receptor subunits in neurons in the periaqueductal gray matter in female Wistar rats. J Comp Neurol 486:89–97
Harney SC, Frenguelli BG, Lambert JJ (2003) Phosphorylation influences neurosteroid modulation of synaptic GABA (A) receptors in rat CA1 and dentate gyrus neurones. Neuropharmacology 45:873–883
Hevers W, Luddens H (1998) The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol 18:35–86
Hsiao CC, Liu CY, Hsiao MC (2004) No correlation of depression and anxiety to plasma estrogen and progesterone levels in patients with premenstrual dysphoric disorder. Psychiatry Clin Neurosci 58:593–599
Korneyev A, Costa E (1996) Allopregnanolone (THP) mediates anesthetic effects of progesterone in rat brain. Horm Behav 30:37–43
Kouri EM, Halbreich U (1998) Hormonal treatments for premenstrual syndrome. Drugs Today 34:603–610
Le Melledo JM, Van Driel M, Coupland NJ, Lott P, Jhangri GS (2000) Response to flumazenil in women with premenstrual dysphoric disorder. Am J Psychiatry 157:821–823
Leidenheimer NJ, Chapell R (1997) Effects of PKC activation and receptor desensitization on neurosteroid modulation of GABA (A) receptors. Brain Res Mol Brain Res 52:173–181
Lovick TA, Griffiths JL, Dunn SM, Martin IL (2005) Changes in GABA(A) receptor subunit expression in the midbrain during the oestrous cycle in Wistar rats. Neuroscience 131:397–405
Maguire JL, Stell BM, Rafizadeh M, Mody I (2005) Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci 8:797–804
Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM (1986) Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 232:1004–1007
Menard J, Treit D (2001) The anxiolytic effects of intra-hippocampal midazolam are antagonized by intra-septal l-glutamate. Brain Res 888:163–166
Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi Z-P, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Zhiwei L, DeLorey TM, Olsen RW, Homanics G (1999) Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor delta subunit knockout mice. PNAS 96:12905–12910
Monteleone P, Luisi S, Tonetti A, Bernardi F, Genazzani AD, Luisi M, Petraglia F, Genazzani AR (2000) Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol 142:269–273
Moran MH, Goldberg M, Smith SS (1998) Progesterone withdrawal II: insensitivity to the sedative effects of a benzodiazepine. Brain Res 807:91–100
Overpeck JG, Colson SH, Hohmann JR, Applestine MS, Reilly JF (1978) Concentration of circulating steroids in normal prepubertal and adult male and female humans, chimpanzees, rhesus monkeys, rats, mice, and hamsters: a literature survey. J Toxicol Environ Health 4:785–803
Pearlstein T, Yonkers KA, Fayyad R, Gillespie JA (2005) Pretreatment pattern of symptom expression in premenstrual dysphoric disorder. J Affect Disord 85:275–282
Pellow S, Chopin P, File SE, Briley M (1995) Validation of open:closed arm entries in an elevated plus maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167
Rapkin A (2003) A review of treatment of premenstrual syndrome and premenstrual dysphoric disorder. Psychoneuroendocrinology 28:39–53
Rapkin AJ, Morgan M, Goldman L, Brann DW, Simone D, Mahesh VB (1997) Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol 90:709–714
Reddy DS, Kim HY, Rogawski MA (2001) Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia 42:328–336
Rodbard D, Hutt DM (1974) Statistical analysis of radioimmunoassay and immunoradiometric assays: a generalized, weighted iterative, least squares method for logistic curve fitting. International Atomic Energy Agency (ed) pp 209–223 (Ref Type: Generic)
Schmidt P, Purdy RH, Moore PH Jr, Paul S, Rubinow D (1994) Circulating levels of anxiolytic steroids in the luteal phase in women with premenstrual syndrome and in control subjects. J Clin Endocrinol Metab 79:1256–1260
Schmidt P, Nieman L, Danaceau M, Adams L, Rubinow D (1998) Differential behavioral effects of gonadal steroids in women with premenstrual syndrome. N Engl J Med 338:209–216
Seippel L, Backstrom T (1998) Luteal-phase estradiol relates to symptom severity in patients with premenstrual syndrome. J Clin Endocrinol Metab 83:1988–1992
Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JMH, Li X (1998a) GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature 392:926–929
Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu F-C (1998b) Withdrawal from 3α-OH-5α-pregnan-20-one withdrawal using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor α4 subunit in association with increased anxiety. J Neurosci 18:5275–5284
Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wasserman EJ (2003a) Abnormal luteal phase excitability of the motor cortex in women with premenstrual syndrome. Biol Psychiatry 54:757–762
Smith MJ, Schmidt PJ, Rubinow DR (2003b) Operationalizing DSM-IV criteria for PMDD: selecting symptomatic and asymptomatic cycles for research. J Psychiatr Res 37:75–83
Smythe JW, McCormick CM, Rochford J, Meaney MJ (1994) The interaction between prenatal stress and neonatal handling on nociceptive response latencies in male and female rats. Physiol Behav 55:971–974
Steiner M, Streiner DL, Steinberg S, Stewart D, Carter D, Berger C, Reid R, Grover D (1999) The measurement of premenstrual mood symptoms. J Affect Disord 53:269–273
Stell BM, Brickley SG, Tang CY, Farrant M, Mody I (2003) Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABA-A receptors. Proc Natl Acad Sci U S A 100:14439–14444
Sundstrom I, Nyberg S, Backstrom T (1997) Patients with premenstrual syndrome have reduced sensitivity to midazolam compared to control subjects. Neuropsychopharmacology 17:370–381
Sundstrom I, Andersson A, Nyberg S, Ashbrook D, Purdy RH, Backstrom T (1998) Patients with premenstrual syndrome have a different sensitivity to a neuroactive steroid during the menstrual cycle compared to control subjects. Neuroendocrinology 67:126–138
Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS (2002) Hormonally regulated alpha(4)beta(2)delta GABA(A) receptors are a target for alcohol. Nat Neurosci 5:721–722
Sur C, Farrar S, Kerby J, Whiting PJ, Atack JR, McKernan RM (1999) Preferential coassembly of alpha-4 and delta subunits of the GABA-A receptor in rat thalamus. Mol Pharmacol 56:110–115
Tiemstra JD, Patel K (1998) Hormonal therapy in the management of premenstrual syndrome. J Am Board Fam Pract 11:378–381
Vicini S, Losi G, Homanics GE (2002) GABA (A) receptor delta subunit deletion prevents neurosteroid modulation of inhibitory synaptic currents in cerebellar neurons. Neuropharmacology 43:646–650
Wafford KA, Thompson SA, Sikela J, Wilcox AS, Whiting PJ (1996) Functional characterization of human GABA receptors containing the α4 subunit. Mol Pharmacol 50:670–678
Wei W, Zhang N, Peng Z, Houser CR, Mody I (2003) Perisynaptic localization of delta subunit-containing GABA (A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci 23:10650–10661
Wisden W, Laurie DJ, Monyer H, Seeburg P (1991) Cloning, pharmacological characteristics and expression pattern of the rat GABAA receptor α4 subunit. FEBS Lett 289:227–230
Wohlfarth KM, Bianchi MT, Macdonald RL (2002) Enhanced neurosteroid potentiation of ternary GABA (A) receptors containing the delta subunit. J Neurosci 22:1541–1549
Yonkers KA (1997) Anxiety symptoms and anxiety disorders: how are they related to premenstrual disorders? J Clin Psychiatry 58:62–69
Zhu WJ, Wang JF, Krueger KE, Vicini S (1996) Delta subunit inhibits neurosteroid modulation of GABAA receptors. J Neurosci 16:6648–6656
Acknowledgements
The authors wish to thank Ellen Freeman, M.D. and Peter Schmidt, M.D. for comments on the manuscript. We are grateful to Jeremy Weedon for assistance with the statistical tests. This work was supported by NIH grants DA09618 and AA12958 and a contract from Lundbeck Pharmaceuticals (Copenhagen, Denmark) to SSS. All experiments performed complied with the current laws of the US.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smith, S.S., Ruderman, Y., Frye, C. et al. Steroid withdrawal in the mouse results in anxiogenic effects of 3α,5β-THP: a possible model of premenstrual dysphoric disorder. Psychopharmacology 186, 323–333 (2006). https://doi.org/10.1007/s00213-005-0168-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-0168-3