Summary
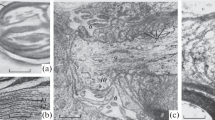
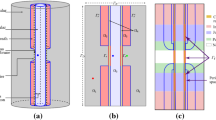
Serially sectioned nodes of Ranvier from nerve fibres 2–20 μm in diameter of feline ventral and dorsal spinal roots were examined electron microscopically, reconstructed to scale and analysed morphometrically. The assumed ‘fresh-state’ value ot several structural variables, considered to be of functional significance, were calculated by the use of compensation factors. The compensated data were plotted against fibre and axon diameters. It was calculated that the membranous area of the ‘fresh-state’ nodal axon segment increased more or less exponentially from less than 5 μm2 to 30 μm2 with increasing fibre diameter (D). Most variables associated with the nodal gap and the Schwann cell initially increased rapidly withD and then levelled out or even decreased in fibres with aD value greater than 8–12 μm. The area open for communication between the nodal axolemma and the endoneurial space was 30–100 times smaller than the membrane area of the nodal axolemma. The volume of the extracellular space in the nodal gap, outside the nodal axolemma, increased linearly from less than 0.1 μm3 to about 0.6 μm3 with increasing fibre size. The Schwann cell membrane area facing the nodal gap outnumbered the membrane area of the nodal axon by 10–15 times in nerve fibres with aD value between 5 and μm. Some functional implications of the ‘fresh-state’ nodal model are discussed.
Similar content being viewed by others
References
Adelman, W. J., Moses, J. &Rice, R. V. (1977) An anatomical basis for the resistance and capacitance in series with the excitable membrane of the squid giant axon.Journal of Neurocytology 6, 621–46.
Arbuthnott, E. R., Boyd, I. A. &Kalu, K. U. (1980) Ultrastructural dimensions of myelinated peripheral nerve fibres in the cat and their relation to conduction velocity.Journal of Physiology 308, 125–57.
Berthold, C. -H. (1968) Ultrastructure of the node-paranode region of mature feline ventral lumbar spinal root fibres.Acta Societatis medicorum upsaliensis 73, suppl. 9, 37–70.
Berthold, C. -H. (1978) Morphology of normal peripheral axons. InPhysiology and Pathobiology of Axons (edited byWaxman, S. G.), pp. 3–64. New York: Raven Press.
Berthold, C. -H. &Rydmark, M. (1983) Electron microscopic serial section analysis of nodes of Ranvier in lumbosacral spinal root of the cat: ultra structural organization of nodal compartments in fibres of different sizes.Journal of Neurocytology 12, 475–505.
Berthold, C. -H., Corneliuson, O. &Rydmark, M. (1982a) Changes in shape and size of cat spinal root myelinated nerve fibres during fixation and Vestopal-W embedding for electron microscopy.journal of Ultrastructure Research 80, 23–41.
Berthold, C. -H., Rydmark, M. &Corneliuson, O. (1983) Estimation of sectioning compression and thickness of ultrathin sections through Vestopal-W embedded cat spinal roots.Journal of Ultrastructure Research 80, 42–52.
Berthold, C.-H., Nilsson, I. &Rydmark, M. (1982c) Axon diameter and myelin sheath thickness in nerve fibres of the ventral root L7 of the adult and the developing cat.Journal of Anatomy, in press.
Brill, M. H., Waxman, S. G., Moore, J. W. &Joyner, R. W. (1977) Conduction velocity and spike configuration in myelinated fibres: computed dependence on internode distance.Journal of Neurology, Neurosurgery and Psychiatry 40, 769–74.
Brismar, T. (1979) Potential clamp experiments on myelinated nerve fibres from alloxan diabetic rats.Acta physiobgica scandinavica 105, 384–6.
Brismar, T. (1980a) Potential clamp analysis of membrane currents in rat myelinated nerve fibres.Journal of Physiology 298, 171–84.
Brismar, T. (1980b) Potential clamp of demyelinated rat nerve fibres.Acta physiologica scandinavica 110, 439–40.
Brismar, T. (1981a) Electrical properties of isolated demyelinated rat nerve fibres.Acta physiologica scandinavica 113, 161–6.
Brismar, T. (1981b) Specific permeability properties of demyelinated rat nerve fibres.Acta physiokgica scandinavica 113, 167–76.
Brismar, T. &Frankenhaeuser, B. (1981) Potential clamp analysis of mammalian myelinated fibres.Trends in Neurosciences 4, 68–70.
Carlstedt, T. (1980) Internodal length of nerve fibres in dorsal roots of cat spinal cord.Neuroscience Letters 19, 251–6.
Chiu, S. Y. &Ritchie, J. M. (1980) Potassium channels in the paranodal region of acutely demyelinated voltage clamped mammalian myelinated nerve.Journal of Physiology 305, 61P-62P.
Chiu, S. Y. &Ritchie, J. M. (1981) Ionic gating currents in mammalian myelinated nerve. InAdvances in Neurology, Vol. 31,Demyelinating Diseases, Basic and Clinical Electrophysiology (edited byWaxman, S. G. andRitchie, J. M.), pp. 313–28. New York: Raven Press.
Chiu, S. Y. &Ritchie, J. M. (1982) Evidence for the presence of potassium channels in the internode of frog myelinated nerve fibres.Journal of Physiology 322, 485–501.
Chiu, S. Y., Ritchie, J. M., Rogart, R. &Stagg, D. (1979) A quantitative description of membrane currents in rabbit myelinated nerve.Journal of Physiology 292, 149–66.
Coggeshall, R. E. (1980) Law of separation of function of the spinal roots:Physiological Reviews 60, 716–55.
Coppin, C. M. L. &Jack, J. J. B. (1972) Internodal length and conduction velocity of cat muscle afferent nerve fibres.Journal of Physiology 222, 91P-93P.
Crank, J. (1956)The Mathematics of Diffusion, pp. 1–347. Oxford: Clarendon Press.
Cullheim, S. (1978) Relations between cell body size, axon diameter and axon conduction velocity of cat α-motoneurons stained with horseradish peroxidase.Neuroscience Letters 8, 17–20.
Cullheim, S. &Kellerth, J. -O. (1978) A morphological study of the axons and recurrent axon collaterals of cat α-motoneurones supplying different functional types of muscle unit.Journal of Physiology 281, 301–13.
Dodge, F. A. (1963)A Study of Ionic Permeability Changes Underlying Excitation in Myelinated Nerve Fibres of the Frog. PhD thesis, Rockefeller Institute, New York, pp. 1–120.
Dodge, F. A. &Frankenhaeuser, B. (1958) Membrane currents in isolated frog nerve fibre under voltage clamp conditions.Journal of Physiology 143, 76–90.
Elfvin, L. -G. (1961) The ultrastructure of the nodes of Ranvier in cat sympathetic nerve fibres.Journal of Ultrastructure Research 5, 374–87.
Ellisman, M. H. (1979) Molecular specializations of the axon membrane at nodes of Ranvier are not dependent upon myelination.Journal of Neurocytology 8, 719–35.
Ellisman, M. H., Friedman, P. L. &Hamilton, W. J. (1980) The localization of sodium and calcium to Schwann cell paranodal loops at nodes of Ranvier and calcium to compact myelin.Journal of Neurocytology 9, 185–205.
Erlanger, J. &Blair, E. A. (1938) Comparative observation of motor and sensory fibres with special reference to the repetitiousness.American Journal of Physiology 121, 431–53.
Frankenhaeuser, B. (1952) The hypothesis of saltatory conduction.Cold Spring Harbor Symposia on Quantitative Biology 17, 27–32.
Frankenhaeuser, B. (1962) Potassium permeability in myelinated nerve fibres ofXenopus laevis.Journal of Physiology 160, 54–61.
Frankenhaeuser, B. &Hodgkin, A. L. (1956) The after effects of impulses in the giant nerve fibres of Loligo.Journal of Physiology 131, 341–76.
Frankenhaeuser, B. &Huxley, A. F. (1964) The action potential in the myelinated nerve fibre ofXenopus laevis as computed on the basis of voltage clamp data.Journal of Physiology 171, 302–15.
Freedman, S. D. &Lentz, T. L. (1980) Binding of horseradish peroxidase-α-bungarotoxin to axonal membranes at the node of Ranvier.Journal of Comparative Neurology 193, 179–85.
Friede, R. L. &Bischhausen, R. (1980) The precise geometry of large internodes.Journal of the Neurological Sciences 48, 367–81.
Hess, A. &Lansing, A. J. (1953) The fine structure of peripheral nerve fibres.Anatomical Record 117, 175–200.
Hess, A. &Young, J. Z. (1952) The nodes of Ranvier.Proceedings of the Royal Society B 140, 301–20.
Hora'kova, M., Nonner, W. &StÄmpfli, R. (1968.Proceedings of the International Union of Physiological Sciences 7, 198.
Huxley, A. F. &StÄmpfli, R. (1951) Effect of potassium and sodium on resting and action potentials of single myelinated nerve fibres.Journal of Physiology 112, 496–508.
Kashef, R. (1966).The Node of Ranvier. PhD thesis, University of London, pp. 1–149.
Landon, D. N. (1981) Structure of normal peripheral myelinated nerve fibres. InAdvances in Neurology, Vol. 31,Demyelinating Diseases, Basic and Clinical Electrophysiology (edited byWaxman, S. G. andRitchie, J. M.), pp. 25–49. New York: Raven Press.
Landon, D. N. &Hall, S. (1976) The myelinated nerve fibre. InThe Peripheral Nerve (edited byLandon, D. N.), pp. 1–105. London: Chapman & Hall.
Landon, D. N. &Langley, O. K. (1971) The local chemical environment of the node of Ranvier. A study of cation binding.Journal of Anatomy 108, 419–32.
Landon, D. N. &Williams, P. L. (1963) Ultrastructure of the node of Ranvier.Nature 199, 575–7.
Langley, O. K. (1969) Ion-exchange at the node of Ranvier.Histochemical Journal 1, 295–309.
Langley, O. K. (1973) Local anaesthetics and nodal polyanions in peripheral nerve.Histochemical Journal 5, 79–86.
Langley, O. K. (1979) Histochemistry of polyanions in peripheral nerve. InComplex Carbohydrates of Nervous Tissue (edited byMargolis, R. U. &Margolis, R. K.), pp. 193–207. New York, London: Plenum Press.
Lieberman, A. R. (1976) Sensory ganglia. InThe Peripheral Nerve (edited byLandon, D. N.), pp. 188–278. London: Chapman & Hall.
Light, A. R. &Metz, C. B. (1978) The morphology of the spinal cord efferent and afferent neurons contributing to the ventral roots of the cat.Journal of Comparative Neurology 179, 501–16.
Livingstone, R. B., Pfenninger, K., Moor, H. &Akert, K. (1973) Specialized paranodaland interparanodal glial-axonal junctions in the peripheral and the central nervous system: a freeze-etching study.Brain Research 58, 1–24.
Ljunggren, S. &Lamm, O. (1957) Diffusion from a bottom layer; diffusion with moving boundaries.Acta Chemica Scandinavica 11, 340–59.
Lüttgau, H. C. (1977) New trends in membrane physiology of nerve and muscle fibres.Journal of Comparative Physiology 120, 51–70.
Neumcke, B., Schwartz, W. &StÄmpfli, R. (1980) Differences between K channels in motor and sensory nerve fibres of the frog as revealed by fluctuation analysis.Pflügers Archiv 387, 9–16.
Palti, Y., Moran, N. &Stämpfli, R. (1980) Potassium currents and conductance. Comparison between motor and sensory myelinated fibers.Biophysical Journal 32, 955–66.
Rasminsky, M. &Sears, T. A. (1972) Internodal conduction in undissected demyelinated nerve fibres.Journal of Physiology 227, 323–50.
Ritchie, J. M. &Chiu, S. Y. (1981) Distribution of sodium and potassium channels in mammalian myelinated nerve. InAdvances in Neurology, Vol. 31,Demyelinating Diseases, Basic and Clinical Electrophysiology (edited byWaxman, S. G. andRitchie, J. M.), pp. 329–42. New York: Raven Press.
Robertson, J. D. (1959) Preliminary observations on the ultrastructure of nodes of Ranvier.Zeitschrift für Zellforschung 50, 553–60.
Rosenbluth, J. (1976) Intramembranous particle distribution at the node of Ranvier and adjacent axolemma in myelinated axons of the frog brain.Journal of Neurocytology 5, 731–45.
Rosenbluth, J. (1978) Glial membrane specializations in extra paranodal regions.Journal of Neurocytology 7, 709–19.
Rosenbluth, J. (1981) Freeze-fracture approaches to ionophore localization in normal and myelin-deficient nerves. InAdvances in Neurology, Vol. 31,Demyelinating Diseases, Basic and Clinical Electrophysiology (edited byWaxman, S. G. andRitchie, J. M.), pp. 391–418. New York: Raven Press.
Rushton, W. A. H. (1951) A theory of the effects of fibre size in medullated nerve.Journal of Physiology 115, 101–22.
Rydmark, M. (1981) Nodal axon diameter correlates linearly with internodal axon diameter in spinal roots of the cat.Neuroscience Letters 24, 247–50.
Schmidt, H. &StÄmpfli, R. (1964) Nachweis unterschiedlicher elektrophysiologischer Eigenschaften motorischer und sensibler Nervenfasern des Frosches.Helvetica physiologica et pharmacologica acta 22, C143-C145.
Schnapp, B., Peracchia, C. &Mugnaini, E. (1976) The paranodal axo-glial junction in the central nervous system studied with thin sections and freeze-fracture.Neuroscience 1, 181–90.
Schwartz, M., Ernst, S. A., Siegel, G. J. &Agranoff, B. W. (1981) Immunocytochemical localization of (Na+, K+)-ATPase in the goldfish optic nerve.Journal of Neurochemistry 36, 107–15.
Skoglund, C. R. (1942) The response to linearly increasing currents in mammalian motor and sensory nerves.Acta physiologica scandinavica 4, suppl. 12.
Smith, K. J. &Schauf, C. L. (1981) Size-dependent variation of nodal properties in myelinated nerve.Nature 293, 297–9.
Stämpfli, R. (1952) Bau und Funktion isolierter markhaltiger Nervenfasern.Ergebnisse der Physiologie 47, 70–165.
Stämpfli, R. (1981) Overview of studies on the physiology of conduction in myelinated nerve fibers. InAdvances in Neurology, Vol. 31,Demyelinating Diseases, Basic and Clinical Electrophysiology (edited byWaxman, S. G. andRitchie, J. M.), pp. 11–23. New York: Raven Press.
Stämpfli, R. &Hille, B. (1976) Electrophysiology of the peripheral myelinated nerve. InFrog Neurobiology (edited byLlinas, R. andPrecht, W.), pp. 3–32. Berlin, Heidelberg, New York: Springer-Verlag.
Uhrik, B. &Stämpfli, R. (1981) Ultrastructural observations on nodes of Ranvier from isolated single frog peripheral nerve fibres.Brain Research 215, 93–101.
Vallbo, Å. B. (1964) Accommodation related to inactivation of the sodium permeability in single myelinated nerve fibres fromXenopus laevis.Acta physiologica scandinavica 61, 429–44.
Waxman, S. G. &Foster, R. E. (1980) Ionic channel distribution and heterogeneity of the axon membrane in myelinated fibres.Brain Research Review 2, 205–34.
Wiley, C. A. &Ellisman, M. H. (1980) Rows of dimeric-particles within the axolemma and juxtaposed particles within glia, incorporated into a new model for the paranodal glial-axonal junction at the node of Ranvier.Journal of Cell Biology 84, 261–80.
Williams, P. L. &Hall, S. M. (1971) Prolongedin vivo observations of normal peripheral nerve fibres and their acute reactions to crush and deliberate trauma,Journal of Anatomy 197, 397–408.
Williams, P. L. &Landon, D. N. (1963) Paranodal apparatus of peripheral nerve fibres of mammals.Nature 198, 670–73.
Wood, J. G., Jean, D. H., Whitaker, J. N., McLaughlin, B. J. &Albers, R. W. (1977) Immunocytochemical localization of the sodium, potassium activated ATPase in knifefish brain.Journal of Neurocytology 6, 571–81.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rydmark, M., Berthold, C.H. Electron microscopic serial section analysis of nodes of Ranvier in lumbar spinal roots of the cat: A morphometric study of nodal compartments in fibres of different sizes. J Neurocytol 12, 537–565 (1983). https://doi.org/10.1007/BF01181523
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01181523